- Title
-
Schwann cell myelination requires Dynein function
- Authors
- Langworthy, M.M., and Appel, B.
- Source
- Full text @ Neural Dev.
|
dync1h1 -deficient larvae express mbp RNA and protein in the central nervous system (CNS) but not the peripheral nervous system (PNS). (A, B) mbp RNA expression in the ventral spinal cord (white arrowhead), along motor nerves (mn, asterisks) and at the posterior lateral line nerve (pLLn, black arrowhead) of wild-type larvae. (C) Immunohistochemistry reveals Mbp (green) associated with motor nerves marked by Acetylated Tubulin staining (red, brackets). Arrows indicate prominent Mbp in the ventral spinal cord. The inset shows Mbp expression, alone, of the portion of the motor nerve, indicated by the dashed box. (D, E) mbp RNA expression in the spinal cord but not at the motor nerves or the pLLn of dync1h1 mutant larvae. (F) Mbp is absent from motor nerves and reduced in the ventral spinal cord of a mutant larva. The inset shows Mbp labeling, alone, of the portion of the motor nerve indicated by the dashed box. (G) pLLn of a wild-type larvae injected with dync1h1 antisense MO lacks mbp RNA expression. Panels A, B, D, E and G show whole 4.25 days post fertilization (dpf) larvae at the level of the mid-trunk with anterior to the left and dorsal to the top. Panels C and F show transverse sections through the level of the trunk with dorsal up. Scale bars equal 20 μm (A, B, D, E, G), 40 μm (C, F) and 50 μm for the insets. |
|
Schwann cells are present at peripheral nerves in dync1h1 mutant larvae. (A, B) Images of transverse sections at the level of the mid-trunk of 3 dpf wild-type (A) and dync1h1 mutant (B) larvae labeled with antibodies to reveal Sox10 (red) and acetylated tubulin (green), which mark Schwann cells and axons, respectively. Schwann cells (arrows) are close to axons of motor nerves (mn) and the posterior lateral line nerve (pLLn). Insets are higher magnification views of motor nerves indicated by the dashed boxes. (C, D) Quantification of Schwann cells associated with motor roots (C) and pLLn (D). Significance of differences in Sox10+ Schwann cell numbers were calculated using nonparametric Mann–Whitney U analysis. Error bars represent standard error of the mean (SEM). Scale bar equals 80 μm for the main panel and 40 μm for the insets. EXPRESSION / LABELING:
|
|
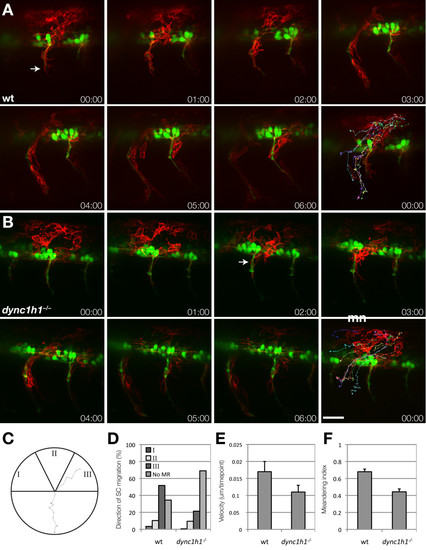
Schwann cells migrate to motor axons in dync1h1 mutant larvae. (A, B) Frames from time-lapse movies of wild-type (A) and dync1h1 mutant (B) larvae carrying Tg(sox10:mRFP) and Tg(mnx1:GFP) transgenes to mark Schwann cells (red, arrow) and motor axons (green). Each sequence starts at 20 hours post fertilization (hpf) and elapsed time is shown in each panel. The final panel in each series shows tracks plotted for the migration of ten RFP+ Schwann cells over 6 hours, with 10 time points per hour. Images shown are lateral views of mid-trunk spinal cord with dorsal up. (C-F) Quantification of Schwann cell progenitor migration. The migration of ten sox10:mRFP+ Schwann cells were tracked over 6 hours with 10 time points per hour. (C) Schwann cell trajectories were assigned a direction of movement based on their starting and ending positions where the apex was assigned as the motor root exit point. I corresponds to anterior to posterior migration, II corresponds to dorsal to ventral migration, and III indicates posterior to anterior Schwann cell migration. A representative track from a wild-type embryo is displayed. (D) Quantification of Schwann cell trajectories from three embryos (30 Schwann cells) indicates that migration to the motor root is reduced but not eliminated in dync1h1 mutants. Categories I, II and III correspond to the diagram in panel C. No MR represents the Schwann cells that did not reach the motor root. (E) Schwann cell velocity is reduced in dync1h1/ embryos (P = 0.004). (F) Schwann cell meandering index, calculated by the displacement from the origin by the track length, is reduced in dync1h1/ embryos (P < 0.0001) indicating less directed movement. P-values were calculated for each track using nonparametric Mann–Whitney U-test statistical analysis. Error bars represent standard error of the mean (SEM). Scale bar equals 40 μm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
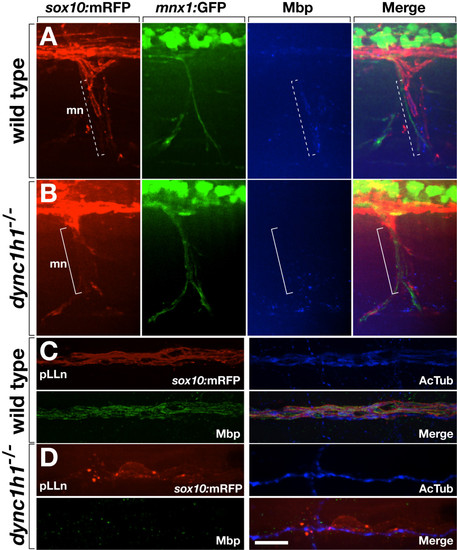
Loss of dync1h1 function disrupts axon wrapping and Mbp expression by Schwann cells. (A, B) Wild-type and dync1h1 mutant larvae expressing sox10:mRFP (red) and mnx1:GFP (green) and labeled by anti-Mbp antibody (blue). Tube-like Mbp+ Schwann cells are evident at some axons of the motor nerve (mn) in wild type (dashed bracket) whereas Mbp– Schwann cells appear more loosely associated with axons in the mutant (solid bracket). (C, D) Wild-type and dync1h1 mutant larvae expressing sox10:mRFP (red) and labeled by anti-Mbp (green) and anti-acetylated tubulin (AcTub, blue) antibodies. Mbp+ Schwann cells ensheath pLLn axons in wild-type. By contrast, RFP+ Schwann cells associated with the pLLn in dync1h1/ mutants do not express Mbp and appear loosely associated with axons. All panels show lateral views of 6 dpf wild-type and dync1h1/ larvae with anterior to the left and dorsal to the top. Scale bars equal 20 μm (A, B) and 10 μm (C, D). |
|
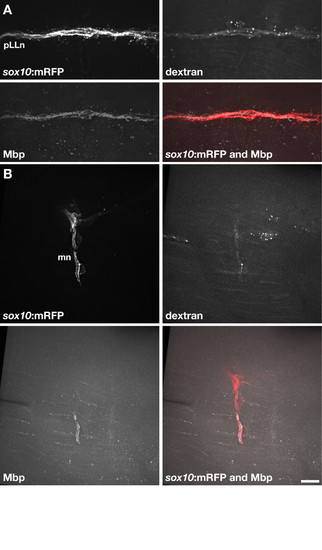
Wild-type Schwann cells express Mbp when associated with dync1h1 mutant axons. (A) Transplanted wild-type Alexa488-dextran+sox10:mRFP+ Schwann cells associated with pLLn axons of a 5 dpf dync1h1 mutant host larva. The transplanted Schwann cells expressed Mbp, detected by immunohistochemistry. (B) Two transplanted wild-type Alexa488-dextran+sox10:mRFP+ Schwann cells associated with motor axons of a dync1h1 mutant host larva. One of the two transplanted Schwann cells expressed Mbp. Scale bar equals 10 μm. |
|
dync1h1 function is required in Schwann cells for robust Mbp expression. (A) Example of dync1h1/sox10:mRFP+ Schwann cells (red) expressing Mbp (blue) at low or undetectable levels when associated with transplanted wild-type elavl3:Kaede+ pLLn axons (green). (B) Example of dync1h1/sox10:mRFP+ Schwann cells expressing detectable levels of Mbp when associated with transplanted wild-type elavl3:Kaede+ pLLn axons. (C) Example of transplanted wild-type Schwann cells expressing Mbp when associated with transplanted wild-type pLLn axons in a dync1h1 mutant host larva. Scale bar equals 10 μm. |
|
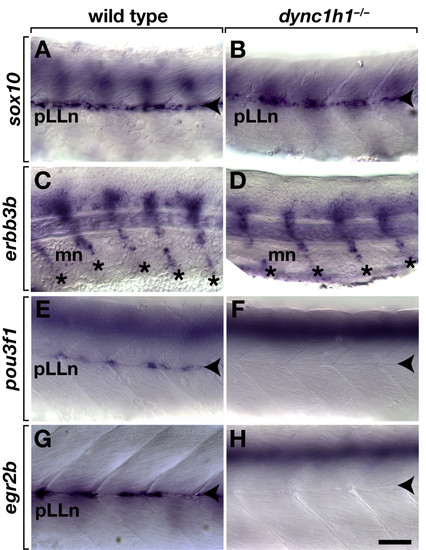
Schwann cells require dync1h1 function to express transcription factors necessary for myelination.sox10 expression is similar at the pLLn (arrowheads) in 52 hours post fertilization (hpf) wild-type (A) and dync1h1 (B) mutant embryos. Schwann cells associated with motor nerves (mn, asterisks) express erbb3b in both wild-type siblings (C) and dync1h1 mutant (D) embryos 52 hpf. Whereas 52 hpf wild-type embryos express pou3f1 at the pLLn (E), expression is absent or present in only a few cells in dync1h1 mutant embryos (F). At 72 hpf wild-type larva expresses egr2b at the pLLn (G), whereas comparably staged dync1h1 mutant larva expresses egr2b in few or no cells (H). Images show lateral views of whole embryos and larvae focused on the mid trunk, with anterior to the left and dorsal up. Scale bar equals 40 μm. EXPRESSION / LABELING:
|
|
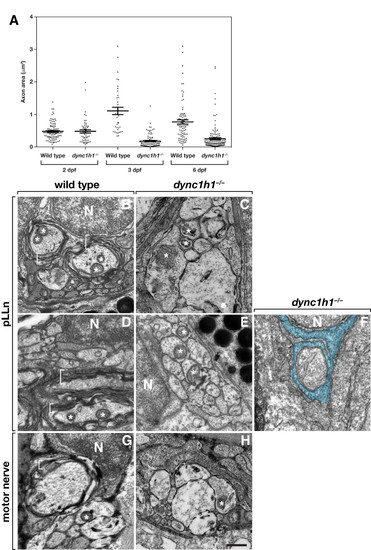
Dync1h1 is required for the formation of myelinating Schwann cells. (A) Scatter plot analysis of axon area. Each point represents one axon. Horizontal bars indicate mean axon area with SD for each group. Panels B-F show transmission electron microscopy (TEM) images of transverse sections through the pLLn, and panels G and H show coronal sections through motor nerves. At 3 days post fertilization (dpf), wild-type axons are loosely wrapped by multiple layers of myelin membrane (brackets) (B) whereas in a dync1h1 mutant larva axons are not ensheathed by myelin (C). By 6 dpf myelin membrane is more compact in a wild-type larva (D) but still absent from a mutant larva (E). Panel F shows an axon wrapped by a single turn of loosely organized Schwann cell membrane (false colored blue). At 4 dpf, myelin ensheaths axon (bracket) of wild-type larvae (G) but is absent from motor axons of a dync1h1 mutant larva (H). Asterisks mark mitochondria, which in mutant axons appear swollen and abnormally shaped. N, Schwann cell nucleus. Scale bar, 0.5 μm. PHENOTYPE:
|
|
Treatment with forskolin rescues peripheral myelin gene expression in dync1h1 mutant larvae. Wild-type larvae treated with forskolin express egr2b (A) and mbp (B, C) RNA normally at motor nerves (mn, asterisks) and the pLLn (arrowhead). dync1h1 mutant larvae treated with forskolin express egr2b (D) and mbp (E, F) RNA indistinguishably from wild type. In forskolin-treated dync1h1/ larvae, Schwann cells associated with motor nerve (G) and pLLn axons (H) marked by anti-acetylated tubulin staining (red), express Mbp (green). Images in A-F are whole mount views of 3 dpf larvae, anterior to the left and dorsal up. Images in G and H are single z-planes captured from transverse sections at 6 dpf. Dashed line indicates the lateral edge of the fish. Scale bars equal 20 μm (A-F), 40 μm (G) and 5 μm (H). EXPRESSION / LABELING:
|