- Title
-
Rargb regulates organ laterality in a zebrafish model of right atrial isomerism
- Authors
- Garnaas, M.K., Cutting, C.C., Meyers, A., Kelsey, P.B., Harris, J.M., North, T.E., and Goessling, W.
- Source
- Full text @ Dev. Biol.
|
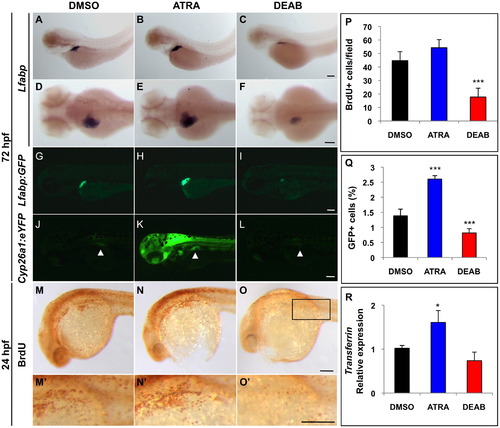
Retinoic acid signaling enhances embryonic liver development. (A–F) In situ hybridization for lfabp at 72 hpf reveals differences in liver size after chemical exposure at 18 hpf. ATRA treatment leads to greater lfabp expression, whereas DEAB treatment leads to reduced lfabp expression. First row, lateral view. Second row, dorsal view. (G–I) Fluorescence microscopy of lfabp:GFP reporter fish at 72 hpf confirms in situ hybridization results in (A–F). (J–L) Fluorescence microscopy of cyp26a1:eYFP reporter fish at 72 hpf demonstrates changes in RA signaling activity in chemically treated embryos. Arrowheads highlight the liver. (M–O) BrdU whole mount immunostaining of 24 hpf embryos demonstrates that ATRA-treated embryos show higher levels of proliferation in the liver primordium (black rectangle) compared to DEAB and DMSO-treated controls. (M2–O2) Enlarged images of (M–O) illustrating individual proliferating cells in the liver primordium. (P) Quantification of the number of BrdU+ cells/field (black rectangle in O). (Q) FACS quantification of percent GFP+ cells in chemically treated lfabp:GFP embryos reveals that ATRA-treated embryos contain more hepatocytes and DEAB-treated embryos contain fewer hepatocytes than DMSO-treated controls. (R) qPCR analysis reveals differences in the relative expression of the hepatocyte marker transferrin in ATRA and DEAB-treated embryos at 72 hpf compared to DMSO-treated controls (where control expression levels were normalized to 1.0). Scale bars: 100 ¼m. EXPRESSION / LABELING:
PHENOTYPE:
|
|
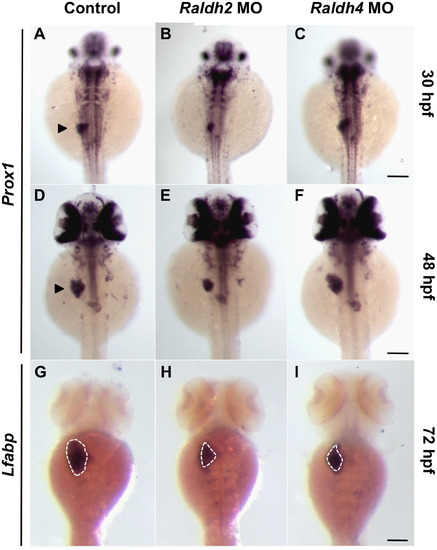
Raldhs 2 and 4 temporally regulate liver development. (A–I) Wild type embryos were injected with raldh2 or raldh4 MO at the 1-cell stage, and liver development was assessed over the next 72 h. In situ hybridization for the hepatoblast marker prox1 at 30 hpf (A–C) and 48 hpf (D–F) and hepatocyte marker lfabp at 72 hpf (G–I) demonstrates distinct temporal requirements for Raldh2 and Raldh4 during liver development. Arrowheads denote the liver primordium, and the dotted lines outline the liver. Scale bars: 100 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
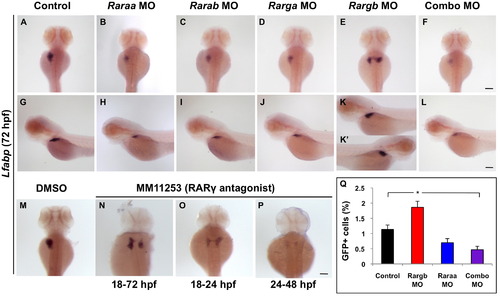
Retinoic acid receptors differentially impact liver development and laterality. (A–L) Lfabp expression at 72 hpf in RAR morphant embryos. Loss of RARs raraa, rarab, and rarga leads to a reduction in liver size (A–D and G–J), whereas loss of rargb causes embryos to develop bilateral livers (E, K and K2). Embryos simultaneously injected with a lower dose of all four RAR MOs develop small livers or none at all (F and L). (M–P) Embryos treated with the RARγ antagonist MM11253 over the time points given develop bilateral livers, confirming genetic knockdown data. (Q) FACS quantification of percent GFP+ cells in lfabp:GFP RAR morphant embryos reveals that rargb morphant embryos with bilateral livers contain significantly more GFP+ hepatocytes than control siblings. Embryos injected with raraa, rarab, rarga, and rargb in combination have roughly half as many GFP+ hepatocytes as control embryos. Scale bars: 100 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
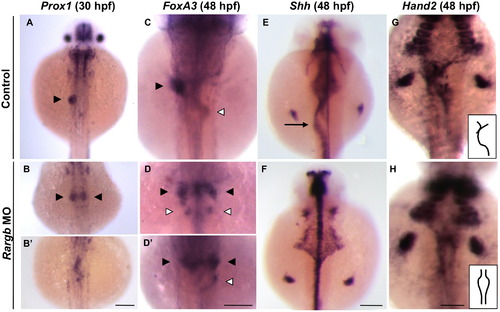
Rargb knockdown impacts the left–right asymmetry of visceral organs. (A and B) Prox1 expression demonstrates that hepatic progenitors normally migrate to the left of the midline at 30 hpf (A, black arrowhead). Loss of rargb results in bilateral (B) or midline (B2) hepatoblast populations. (C-D) In situ hybridization for the pan-endodermal marker foxA3 at 48 hpf demonstrates that loss of rargb results in bilateral livers (black arrowheads) that are sometimes accompanied by bilateral pancreata (white arrowheads). (E and F) Rargb morphants develop gut looping abnormalities (arrow), as demonstrated by shh expression at 48 hpf (E and F). (G and H) Hand2 expression at 48 hpf demonstrates aberrant LPM migration in rargb morphants (H). Insets in (G and H) highlight the differences in LPM phenotypes. Scale bars: 100 ¼m. |
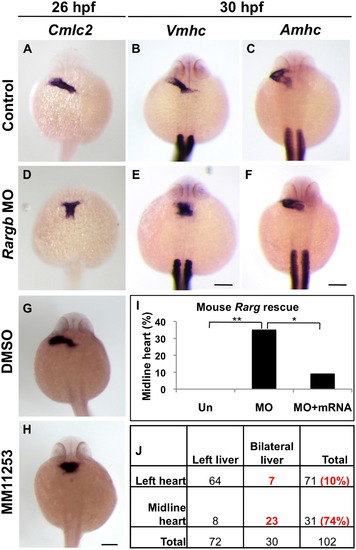
|
Midline hearts and bilateral livers correlate in rargb morphants. (A–F) Control embryos develop leftward jogging hearts as shown by the pan-cardiomyocyte marker cmlc2 at 26 hpf (A) and chamber-specific markers vmhc and amhc at 30 hpf (B and C), whereas rargb morphants develop midline ventricles (D and E). (G and H) Embryos treated with the RARγ antagonist MM11253 develop midline hearts, confirming genetic knockdown data. (I) RARγ function is evolutionarily conserved. The midline heart phenotype of rargb morphant embryos is rescued by injection of mouse Rarg mRNA. (J) Midline hearts and bilateral livers correlate in the same embryo. Cmlc2:GFP reporter fish injected with rargb MO were sorted by heart phenotype at 30 hpf, then liver laterality was assessed at 72 hpf by lfabp expression. Scale bars: 100 µm. |
|
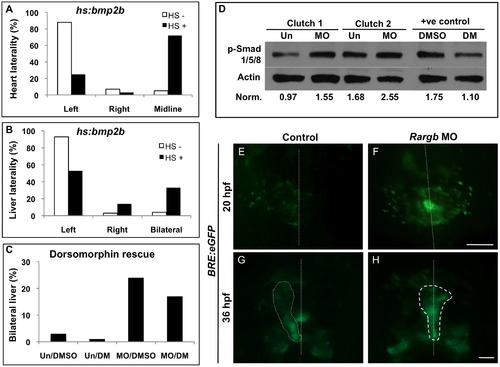
Rargb negatively regulates Bmp activity and localization in vivo. (A and B) Overexpression of Bmp in heat shock inducible hs:bmp2b embryos results in midline hearts (A) and bilateral livers (B), paralleling the phenotype of rargb morphants. (C) Treatment of rargb morphant embryos with the Bmp inhibitor dorsomorphin results in a reduction in the number of bilateral livers compared to DMSO-treated control rargb morphants. (D) Western blot for Bmp effectors phospho-Smads 1/5/8 in uninjected control (Un) and rargb MO-injected embryos (MO) at 18 hpf. Band density values normalized to Actin demonstrate that loss of rargb results in increased expression of p-Smads 1/5/8. Embryos treated with the Bmp inhibitor dorsomorphin (DM) show reduced p-Smad 1/5/8 expression compared to DMSO-treated controls. (E–H) Fluorescence microscopy of Bmp response element reporter fish BRE:eGFP. At 20 hpf, control embryos display greater Bmp activity on the left side (E), whereas rargb morphants display symmetrical Bmp activity (F). Altered Bmp localization at 20 hpf corresponds to heart laterality defects at 36 hpf in rargb morphants (H). White dotted lines denote the midline (E and F) or outline the heart tube (G and H). Scale bars: 100 µm. EXPRESSION / LABELING:
PHENOTYPE:
|
|
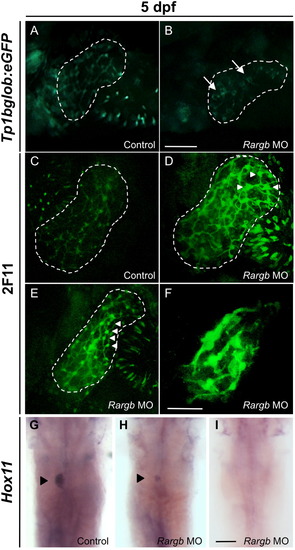
Rargb morphants develop biliary defects. (A and B) Fluorescence microscopy of Notch reporter fish tp1bglob:eGFP at 5 dpf. Rargb morphant embryos develop defective bile duct architecture (B, arrows) compared to the normal latticework appearance of the control embryo′s biliary tree (A). (C–F) Confocal microscopy of 2F11 whole mount immunostained embryos at 5 dpf. Rargb morphant embryos display enlarged cell bodies (D and E, white arrowheads) and reduced branching of intrahepatic bile duct networks (F) compared to control embryos (C). White dotted lines outline the liver. Images are taken at the same magnification. (G–I) In situ hybridization for hox11 at 5 dpf. Rargb morphants display small (H) or absent (I) spleens compared to control embryos (G). Black arrowheads denote the spleen. Scale bars: 50 ¼m. |
|
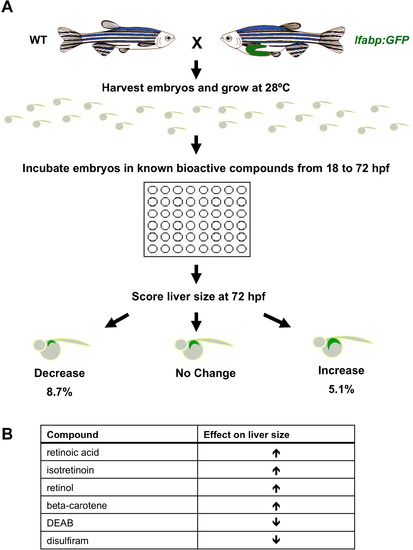
A chemical genetic screen identifies retinoic acid as a regulator of embryonic zebrafish liver development. A chemical genetic screen identifies retinoic acid as a regulator of embryonic zebrafish liver development. (A) Chemical genetic screen workflow. Wild type (WT) or lfabp:GFP transgenic reporter fish were exposed to individual compounds from a library of 2640 known bioactives over the course of embryonic liver development (18-72 hpf). Alterations in liver size were detected by fluorescence microscopy and in situ hybridization for lfabp. (B) Eight hits corresponding to six compounds were identified in the screen. Retinoic acid, isotretinoin, retinol, and beta-carotene increased liver size, whereas RA pathway inhibitors diethylaminobenzaldehyde (DEAB) and disulfiram decreased liver size. |
|
The pan-RAR antagonist AGN193109 reduces liver size and hepatic proliferation. (A-B) Embryos treated with the RAR antagonist AGN193109 from 18-24 hpf develop smaller livers compared to DMSO-treated controls as shown by lfabp expression at 72 hpf. Lateral views. (C) Quantification of the number of proliferating BrdU+ cells in the area of the liver primordium (see Figure 1, black rectangle) in AGN193109-treated embryos at 24 hpf. (D) FACS quantification of percent GFP+ cells in chemically treated lfabp:GFP embryos reveals that AGN193109-treated embryos contain fewer hepatocytes than DMSO-treated controls. |
|
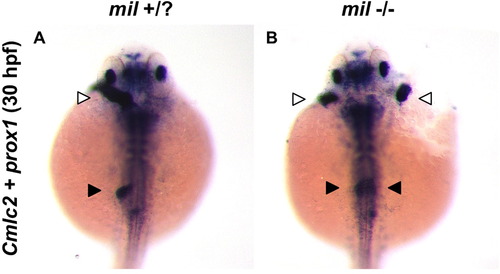
Cardia bifida mutants miles apart (mil) develop bilateral livers. (A-B) In situ hybridization at 30 hpf for heart and hepatoblast markers cmlc2 and prox1, respectively, demonstrates that cardia bifidia mutants mil develop two hearts (white arrowheads) and bilateral livers (black arrowheads). PHENOTYPE:
|
|
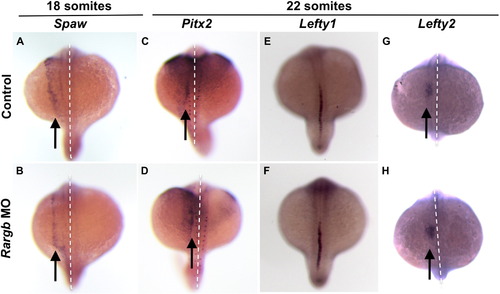
Loss of rargb does not affect Nodal signaling Rargb knockdown does not affect normal left-sided expression of Nodal signals spaw (A, B), pitx2 (C, D), or lefty2 (G, H) (arrows) or the molecular midline barrier lefty1 (E, F). White dotted lines denote the midline. PHENOTYPE:
|
|
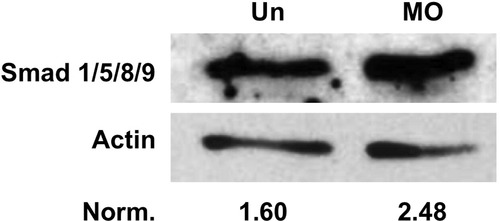
Levels of Smad 1/5/8/9 proteins are increased in rargb morphants. Western blot for Smad 1/5/8/9 on whole embryo lysates at 18 hpf. Loss of rargb (MO) results in an increase in Smad protein levels compared to uninjected controls (Un). Band density values normalized to Actin demonstrate that loss of rargb results in increased expression of Smads 1/5/8/9. |
|
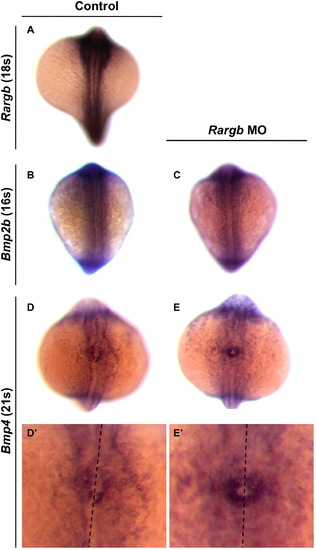
Bmp4 is symmetrically expressed in rargb morphants. In situ hybridization for rargb (A), bmp2b (B, C), and bmp4 expression (D, E) in control and rargb morphant embryos during somitogenesis. (D′, E′) are enlarged images of the cardiac cone of embryos shown in (D, E). Black dotted lines denote the midline. |

Unillustrated author statements PHENOTYPE:
|
Reprinted from Developmental Biology, 372(2), Garnaas, M.K., Cutting, C.C., Meyers, A., Kelsey, P.B., Harris, J.M., North, T.E., and Goessling, W., Rargb regulates organ laterality in a zebrafish model of right atrial isomerism, 178-189, Copyright (2012) with permission from Elsevier. Full text @ Dev. Biol.