- Title
-
Mesogenin causes embryonic mesoderm progenitors to differentiate during development of zebrafish tail somites
- Authors
- Yabe, T., and Takada, S.
- Source
- Full text @ Dev. Biol.
|
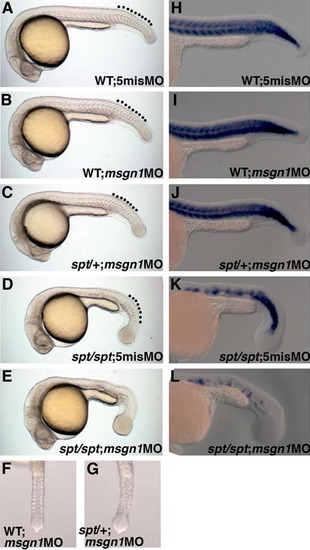
msgn1 and spt are required for the differentiation of tail PSM in zebrafish. (A)-(G) Morphologies of wild-type zebrafish embryos injected with 5mis MO (A) or msgn1 MO (B), spt heterozygous embryo injected with msgn1 MO (C), and spt homozygous embryo injected with 5mis MO (D) or msgn1 MO (E) at 24 hpf. Ventral views of the tailbud region of msgn1 MO-injected wild-type (F) and spt heterozygous (G) embryos are also shown. Broken lines indicate the tail somites. (H)-(L) Defective tail myogenesis in msgn1;spt double-deficient embryos. Expression of myod, a myogenic gene, in the tail region of the msgn1-injected spt mutant was severely reduced (L) compared with that in the wild-type or single-deficient embryo (H)-(K). Embryos were fixed at 24 hpf for in situ hybridization. |
|
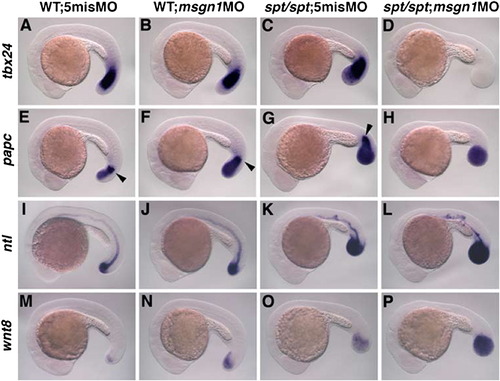
Marker analysis of msgn1;spt double-deficient embryos. Wild-type embryos injected with 5mis MO (A, E, I, and M) or msgn1 MO (B, F, J, and N) and spt homozygous embryos injected with 5mis MO (C, G, K, and O) or msgn1 MO (D, H, L, and P) were fixed at the 24-somite stage and hybridized with tbx24 (A-D), papc (E-H), ntl (I-L) or wnt8 (M-P) probes. The expression of tbx24 in the PSM is severely reduced in the msgn1 MO-injected spt homozygous embryo (D) compared with that in the wild-type (A), msgn1 MO-injected wild-type (B) or spt single-mutant embryo (C). Similarly, papc-positive migrating cells in the anterior PSM (indicated by arrowheads) were not detected in the msgn1 MO-injected spt homozygous embryo (H). Injection of msgn1 MO into wild-type embryos increased the expression area of ntl and wnt8 in the tailbud (J and N) compared with that for the control MO (I and M). In addition, ntl and wnt8-expressing cells are also increased by msgn1 MO in spt mutant embryos (K, L, O, and P). EXPRESSION / LABELING:
|
|
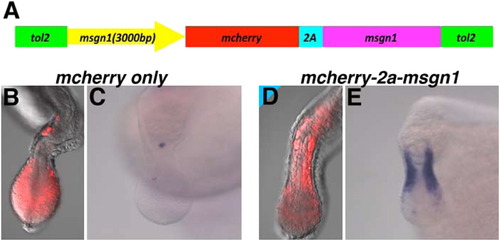
msgn1 expression driven by the 3000-bp msgn1 promoter rescues msgn1 morphant embryos from the PSM phenotype. (A) Schematic representation of DNA construct used in the experiments. The msgn1 ORF was connected to the C-terminus of mCherry via the 2a peptide. The translation of mRNA transcripts from this cDNA is expected not to be inhibited by msgn1 MO, because this MO blocks the translation initiated from the start codon of msgn1 mRNA. This construct was inserted between the tol2-inverted repeats following the 3000-bp msgn1 promoter. (B)-(E) The anterior migration of mCherry-positive cells and tbx24 expression in msgn1;spt double-deficient embryos were rescued by the injection of 25 pg of rescue construct with 50 pg of synthesized tol2 transposase mRNA (D and E, n=48, 100%) but not by injection of the construct without the msgn1 ORF (B and C, n=48, 100%). |
|
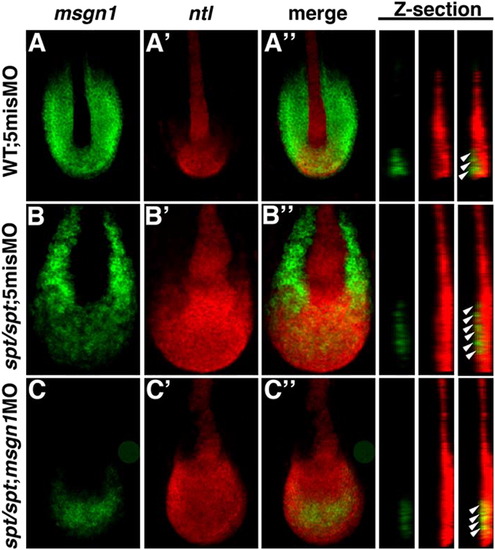
Roles of Msgn1 and Spt is required for PSM differentiation in the maturation zone. In situ hybridization of wild-type embryos injected with 5mis MO (A) and spt mutant embryos injected with 5mis MO (B) or msgn1 MO (C). Probes for msgn1 (green; A, B, C) and ntl (red; A′, B′, C′) were used. Merged images of ntl and msgn1 expression are also shown (A′′, B′′, C′′). Z-stacked images at the midline, indicated by the dotted line in the merged images, are also indicated at the right. White arrowheads indicate the maturation zone where cells express msgn1 and ntl. Embryos were fixed at the 10-somite stage. Confocal images were taken of flat mounts after yolk removal. EXPRESSION / LABELING:
|
|
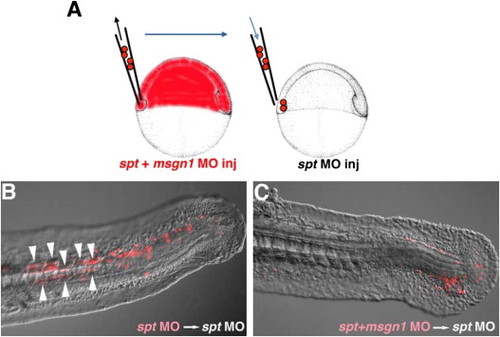
Msgn1 functions in a cell-autonomous manner in tail PSM formation. (A) Schematic representation of the transplantation experiment. Rhodamine-labeled cells from spt MO- or msgn1;spt MO-injected embryos were transplanted to the ventral margin of the spt MO-injected host embryo at the shield stage. (B and C) While the spt single knocked down cells could contribute to the formation of the tail PSM and differentiation of the tail muscle in the host embryo (B, n=34; 71%), spt and msgn1 double knocked down cells remained at the posterior tip of the tail and failed to contribute to formation of the tail muscle (C, n=32; 0%). Arrowheads indicate the transplanted cells that differentiated into tail myofiber-containing cells. |
|
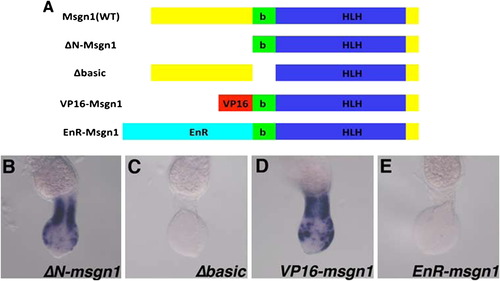
Msgn1 functions as a transcriptional activator in tail PSM formation. (A) Schematic representation of msgn1 mutants. ΔN-Msgn1 lacks most of the N-terminal region, and the Δbasic form of Msgn1 lacks only the basic domain. In VP16-Msgn1 and EnR-Msgn1, the transcriptional activator domain of VP16 and the transcriptional repressor domain of Engrailed of Drosophila melanogaster, respectively, were fused to the N-terminus of the ΔN-Msgn1. These mutant forms were connected to the C-terminal region of the mCherry with the 2a peptide, designed to be expressed under the control of the msgn1 promoter, and injected into spt mutant eggs by using the tol2-based micro-injection system with msgn1 MO. (B)-(E) The ΔN-msgn1 was able to rescue the tbx24 expression in the PSM (B, n=33,100%), whereas the Δbasic form was not (C, n=24,100%). The tbx24 expression in msgn1-injected spt mutant embryos was rescued by the VP16- (D, n=28,100%) but not by the EnR- (E,n=29,100%) Msgn1. |
|
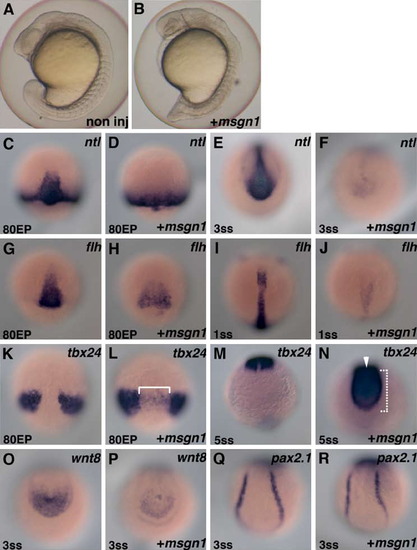
Over-expression of msgn1 can exert PSM-inducing activity. (A and B) Lateral views of control (A) and msgn1 mRNA (200 pg)-injected (B) embryos at the 16-somite stage. (C)-(R) Marker analysis of msgn1 mRNA over-expressed embryo. Control (C, E, G, I, K, M, O, and Q) and msgn1 mRNA-injected (D, F, H, J, L, N, P, and R) zebrafish embryos hybridized with ntl (C-F), flh (G-J), tbx24 (K-N), wnt8 (O and P) or pax2.1 (Q and R) probe. Stage of the embryo is indicated at the left bottom in each photo. Dorsal views of axial region (C, D, G, H, K, and L), tailbud region (E, F, I, J, and M-P) and trunk region (Q and R) are shown. In the axial region, significantly reduced expression of ntl (C and D) and flh (G and H), and ectopic expression of tbx24 (K and L, shown with a bracket) are observed at the mid-gastrulation stage (80% epiboly). Reduced axial expression of ntl (E and F) and flh (I and J), and ectopic axial expression of tbx24 in the posterior tip (bracket) and axial region (arrowhead) (M and N), continued at the early-somite stage (3 somites, 1 somite, and 5 somites, respectively). The expressions of ntl (E and F) and wnt8 (O and P) in the tailbud were severely reduced in the msgn1-injected embryo at the early somite stage (3 somites). The expression of tbx24 was expanded to the posterior tip of the tailbud (M and N). However, pronephric expression of pax2.1 was not affected (Q and R). EXPRESSION / LABELING:
|
|
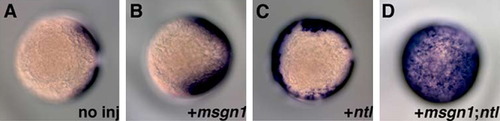
Cooperative roles of msgn1 and ntl in PSM induction. In comparison with no injection (A), injection of 200 pg of msgn1 (B) or 25 pg of ntl (C) mRNA induced ectopic expression of tbx24 in the axial region or margin, respectively, but not in most cells in the animal hemisphere. On the other hand, co-injection of 200 pg of msgn1 and 25 pg of ntl mRNA induced strong expression of tbx24 in the animal hemisphere (D). Embryos were fixed at the 95% epiboly stage for in situ hybridization, and photos were taken from the animal pole of the embryos. |
|
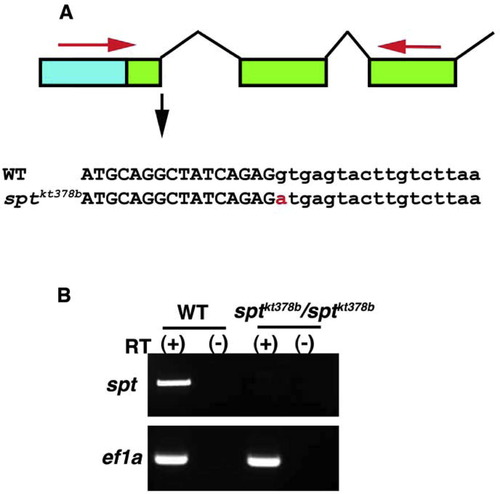
Identification of new spt mutant allele. (A) The sptkt378b allele contains a point mutation in the splicing donor sequence of the 1st intron. Blue box and green boxes indicate the respective 5′ UTR and ORF of spt genes. Large and small characters define the exons and introns, respectively. The red character indicates the point mutation detected in the sptkt378b allele. (B) Correctly spliced spt mRNA is not generated in the sptkt378b allele. RNA from the homozygous sptkt378b and wild-type embryos at the mid-somite stage were used for RT-PCR with primers designed for the 1st exon and 3rd exon of the spt gene (shown by red arrow in "A"). The expected size of PCR product was not amplified from the homozygous embryo. |
|
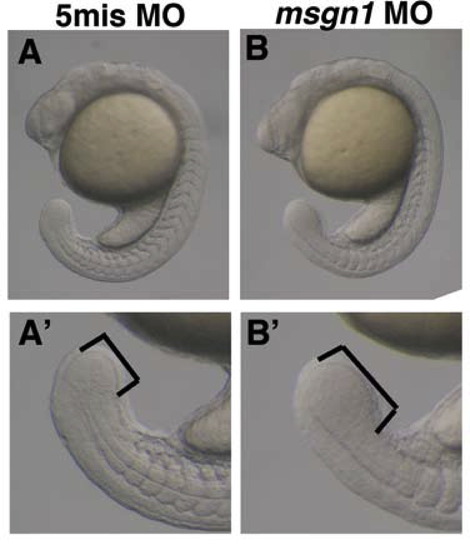
Phenotype of msgn1 MO-injected embryos at the 20-somite stage. In the msgn1 MO-injected embryo, the tailbud is enlarged compared with that of the control embryo (A and B). A′ and B′ are images of tailbuds at higher magnification. |
|
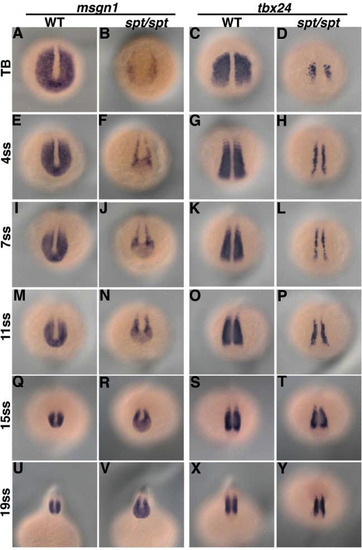
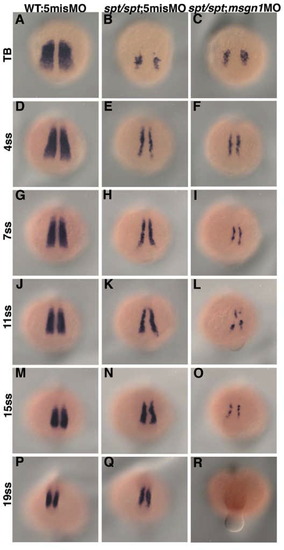
The msgn1 and tbx24 expression in spt mutants. In situ hybridization analysis of msgn1 expression in wild-type (A, E, I, M, Q, and U) and spt mutant (B, F, J, N, R, and V) embryos revealed that the expression of msgn1 was restored through the progressive development during the somite stage. Time courses of tbx24 expression in wild-type (C, G, K, O, S, and X) and spt mutant (D, H, L, P, T, and Y) embryos are also shown. The embryos were fixed at the tailbud stage (A-D) or 4- (E-H), 7- (I-L), 11- (M-P), 15- (Q-T) or 19- (U-Y) somite stage. |
|
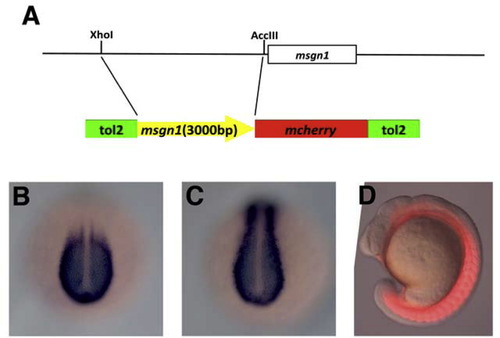
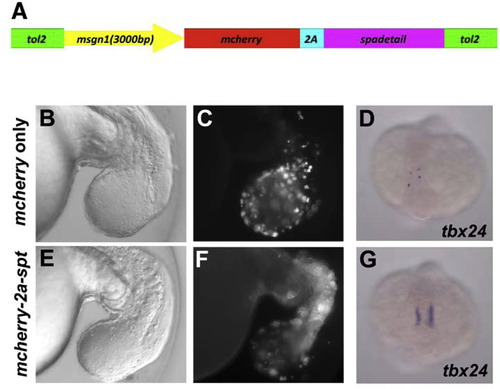
Isolation of msgn1 promoter. Schematic representation of msgn1 promoter isolation. An approx. 3000-bps genomic fragment upstream of then msgn1 coding region was isolated by ScaI and AccIII digestion of a Bac clone. This sequence was fused to the 5′ of mCherry and cloned between the tol2 inverted repeat elements. (B-D) The expression of mCherry in transgenic embryos. The embryos carrying the transgene containing mcherry connected to the down-stream of the msgn1 3000-bp promoter recaptured the endogenous msgn1 expression in the tailbud and the posterior PSM. This DNA element also drove the expression in the anterior paraxial mesoderm and somites, possibly due to the lack of some cis-element required to terminate the transcription at the anterior paraxial mesoderm or because of a difference of mRNA stability between msgn1 and mcherry (B and C). The mCherry protein was also detected in the paraxial mesoderm (D). |
|
spt expression driven by the msgn1 promoter rescues msgn1;spt double-deficient embryos from the PSM phenotype. (A) Schematic representation of DNA construct used in the experiments. (B-G) Twenty five pg of control construct (B-D) or rescue construct (E-G) were injected into msgn1;spt double-deficient embryos with 50 pg of synthesized tol2 transposase mRNA. Anterior migration of mCherry-positive cells (B, C, E, and F) and tbx24 expression (D and G) were compared in embryos injected with the rescue construct and in control embryos. |
|
Comparison of tbx24 expression between spt single-deficient and msgn1;spt double-deficient embryos. Tbx24 expression in control MO-injected wild-type embryos (A, D, G, J, M, and P), control MO-injected spt mutant (B, E, H, K, N, and Q) and msgn1 MO-injected spt mutant (C, F, I, L, O, and R) is shown. In the spt single-deficient embryo, tbx24 expression was gradually restored during the somite stage. On the other hand, in msgn1;spt double-deficient embryos, the tbx24 expression was low through most of the somite stages and finally eliminated at the late somite stage. The embryos were fixed at the tailbud stage (A-C) or at the 4- (D-F), 7- (G-I), 11- (J-L), 15- (M-O) or 19- (P-R) somite stage. |
|
The expression of sox2 in msgn1;spt double-deficient embryos. Wild-type embryos injected with 5mis MO (A) or msgn1 MO (B) and spt homozygous embryos injected with 5mis MO (C) or msgn1 MO (D) were fixed at the 24 hpf and hybridized with sox2. |
Reprinted from Developmental Biology, 370(2), Yabe, T., and Takada, S., Mesogenin causes embryonic mesoderm progenitors to differentiate during development of zebrafish tail somites, 213-222, Copyright (2012) with permission from Elsevier. Full text @ Dev. Biol.