- Title
-
Zebrafish Cxcr4a determines the proliferative response to Hedgehog signalling
- Authors
- Stückemann, T., Wegleiter, T., Stefan, E., Nägele, O., Tarbashevich, K., Böck, G., Raz, E., and Aanstad, P.
- Source
- Full text @ Development
|
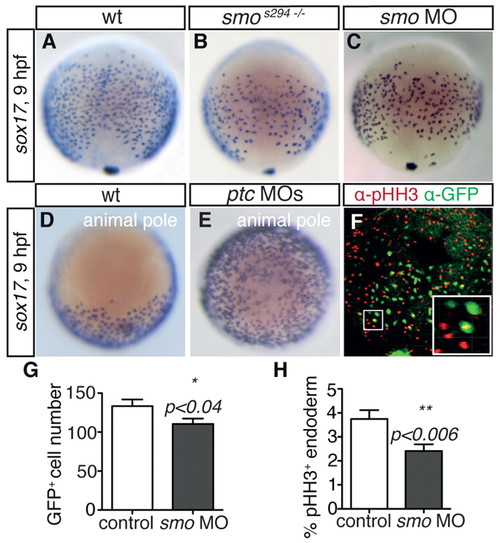
smos294 acts as a dominant-negative and reveals a requirement for Hh signalling in endodermal cell proliferation. (A-E) In situ hybridisation for sox17 at 9 hpf showing (A-C) dorsal view and (D,E) animal pole view. smos294 mutant (B) and smo morphant (C) zebrafish embryos showed a reduction in sox17-positive endodermal cells compared with wt siblings (A). Activation of Hh signalling by injection of ptc1 and ptc2 morpholinos (ptc MOs) (E) caused an increased number of sox17-positive cells compared with controls (D). See also supplementary material Fig. S1. (F) Presence of pHH3 (red) in Tg(sox17:EGFP)-positive endodermal cells (green) at 9 hpf (dorsal view). Inset illustrates higher magnification view of one pHH3-positive endodermal cell. (G,H) Quantification of Tg(sox17:EGFP)-positive (G) and Tg(sox17:EGFP) and pHH3 double-positive (H) cells in control and smo morphant embryos. Bars show mean number of cells (G) or mean percentage of double-positive cells (H); error bars indicate s.e.m. n=18 embryos for control and n=23 for smo morphant embryos in three independent experiments. |
|
Hh promotes cell cycle progression in the endoderm but not in non-endodermal cells. (A) Outline of transplant assay. Rhodamine-dextran-positive donor cells are transplanted into host zebrafish embryos and counted at the beginning (6 hpf) and at the end (10 hpf) of gastrulation. The fold change is taken as a measure of donor cell proliferation. (B-D) A host embryo with transplanted control endodermal donor cells (Rhodamine-dextran, red) at 6 hpf (B) and 10 hpf (C). Control endodermal cells double in number during gastrulation and express Tg(sox17:EGFP) at 10 hpf (D). (E,F) Quantification of transplant assay results with injected endodermal donor cells in a wt host background (E) and wt endodermal donor cells in a morphant host background (F). (G) Quantification of transplant assay using injected non-endodermal donor cells in wt host background. (E-G) Bars show mean fold change from at least three independent experiments; error bars show s.e.m. n values for each experiment are given in supplementary material Table S2. ***P<0.001; one-way ANOVA analysis. (H,I) qRT-PCR analysis of FACS sorted Tg(sox17:EGFP)-positive (endoderm, H) and -negative (non-endoderm, I) smo morphant samples compared with uninjected control samples. Bars show mean fold change; error bars indicate s.e.m. |
|
Cxcr4a is required for endodermal cell proliferation and determines the proliferative response to Hh signalling. (A,B) In situ hybridisation for sox17 at 9 hpf in control (A) and cxcr4a morphant (B) zebrafish embryos. (C) Quantification of pHH3 and Tg(sox17:EGFP) double-positive endoderm cells in cxcr4a single-morphant and smo;cxcr4a double-morphant Tg(sox17:EGFP) embryos. Bars show mean percentage of double-positive cells; error bars indicate s.e.m. n=18 (control), n=23 (cxcr4a MO) and n=31 (smo;cxcr4a double MO) embryos in three independent experiments. (D,E) Quantification of transplant assay results using endodermal (D) and non-endodermal (E) donor cells. Bars show mean fold change of three independent experiments. n values are given in supplementary material Table S2. *P<0.05; **, P<0.01; ***P<0.001; Student′s t-test (C) or one-way ANOVA analysis (D,E). |
|
Cxcr4a promotes gli1 expression. (A) Quantification of gli1 mRNA levels in smo and cxcr4a morphant endoderm relative to control. (B) Quantification of gli1 mRNA levels in whole zebrafish embryos relative to controls. (C) In situ hybridisation for ptc1 in control and cxcr4a morphant embryos at 10 hpf. (D) Quantification of ptc1 mRNA levels in whole embryos (10 hpf) injected with cxcr4a mRNA, relative to control. Bars indicate mean fold change; error bars indicate s.e.m. ***P<0.001; ns, not significant; one-way ANOVA analysis. |
|
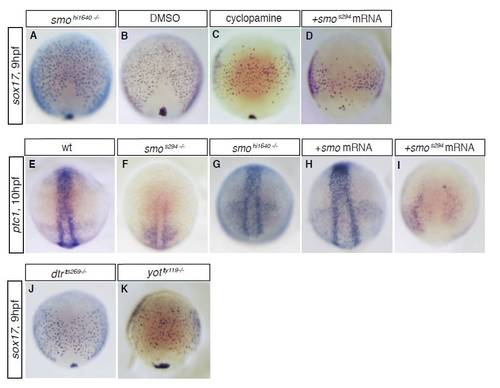
The smos294 allele acts as a dominant-negative. In situ hybridisation for the endodermal marker gene sox17 (A-D,J,K) and the Hh target gene ptc1 (E-I) at 9-10 hpf. (A,B) smohi1640 mutant embryos showed normal expression of sox17 during gastrulation (compare A with B), in contrast to smos294 mutant embryos (see Fig. 1B). This suggests that smos294 acts differently to the hi1640 null allele. (C) To confirm that the s294 allele affects Smo function during gastrulation, we also inhibited Smo function using cyclopamine, and found that cyclopamine treatment phenocopies smos294 mutant embryos. (E-G) To confirm that the endodermal defects we observed in smos294 mutants correlate with defects in Hh pathway target genes, we performed in situ hybridisation for ptc1 and found that smos294 (F), but not smohi1640 (G), showed reduced ptc1 expression at the end of gastrulation (compare with E). These results indicate attenuated Hh signalling in smos294 but not in smohi1640 mutant embryos during gastrula stages. (D,H,I) As smo is maternally provided in zebrafish, this suggests that the smos294 allele might act as a dominant-negative. To test this, we injected mRNA encoding wt smo or smos294 into wt embryos and assessed sox17 and ptc1 expression. Injection of wt smo mRNA does not affect the expression of either sox17 (data not shown) or ptc1 (H). However, injection of smos294 mRNA strongly reduces expression of both sox17 (D) and ptc1 (I). These results suggest that s294 acts as a dominant-negative allele of smo. (J) dtrts269 mutant embryos showed a slight reduction in sox17-positive endodermal cells at the end of gastrulation, suggesting that loss of Gli1 causes a reduction in endodermal cell numbers. (K) yotty119 mutant embryos showed a similar reduction in the number of endodermal cells at the end of gastrulation. As the yotty119 allele acts as a dominant repressor, these results suggest that Gli2/3 is required for gli1 expression and normal endodermal cell number in the gastrula stage embryo. |
|
A proliferation assay based on cell transplantation. Host embryos with donor cells at 6 hpf (A,D) or 10 hpf (B,C,E,F). (A-C) Injection of cas mRNA converts donor cells into endoderm, as indicated by Tg(sox17:EGFP) expression at 10 hpf (C). Injection of smo MO blocks proliferation of endodermal donor cells (A,B). (D-F) Cells from donor embryos that were not injected with cas mRNA do not express Tg(sox17:EGFP) (F), indicating that they become mesodermal and/or ectodermal. |
|
Hh signalling regulates p57 expression in gastrula stage zebrafish embryos. In situ hybridisation for p57 at 9-10 hpf in control (A), cas (B), smo (C) and ptc (D) morphant embryos. (A) High levels of p57 expression were detected in axial mesoderm and additional cell populations. (B) This expression pattern is not changed in cas morphant embryos, which lack endoderm, suggesting that p57-expressing cells are mesodermal and/or ectodermal. (C) In smo morphants, axial expression of p57 persists, although p57 expression is abolished in other domains of the embryo. (D) In ptc morphants, the expression of p57 is expanded. |
|
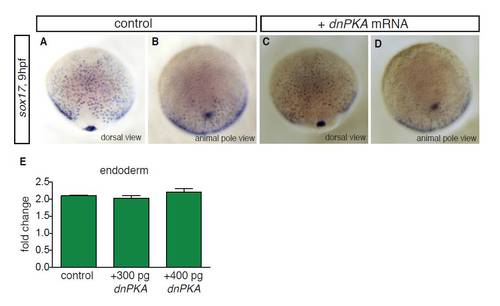
Overexpression of dnPKA does not affect sox17-positive endodermal cell numbers. (A-D) In situ hybridisation for sox17 at 9 hpf in control embryos (A,B) and embryos injected with 200 pg dnPKA mRNA (C,D). (A,C) Dorsal views; (B,D) animal pole views. Although injection of ptc MOs gave an increase in sox17-positive endodermal cells at 9 hpf, no significant increase in endodermal cells was detected in embryos injected with 200 pg dnPKA mRNA (compare D with Fig. 1E), consistent with the lack of increased proliferation in dnPKA-injected endodermal donor cells (see Fig. 6A). (E) Injection of higher amounts of dnPKA mRNA (300 pg and 400 pg) did not significantly change proliferation of endodermal donor cells in the transplant assay, suggesting that the lack of significant effects of dnPKA mRNA injection on endodermal cell proliferation is not due to insufficient amounts of dnPKA. |