- Title
-
Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- Authors
- Stevenson, T.J., Trinh, T., Kogelschatz, C., Fujimoto, E., Lush, M.E., Piotrowski, T., Brimley, C.J., and Bonkowsky, J.L.
- Source
- Full text @ PLoS Genet.
|
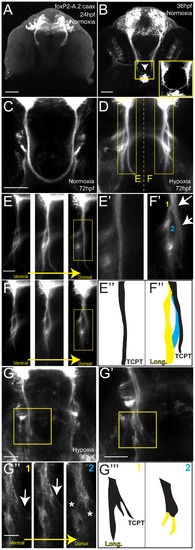
Hypoxia disrupts axon pathfinding in the developing CNS. Confocal images are maximum intensity projections of whole-mount embryos, ventral views, rostral top, scale bars 50 μm. (A, B) 48 hpf embryos, acetylated tubulin immunohistochemistry. (B) Following 1% hypoxia (1% pO2) from 24–36 hpf, anterior commissure and post-optic commissural tracts are disrupted (arrows). (C–I) Tg(foxP2-enhancerA.2:egfp-caax) embryos (abbreviated foxP2-A.2:caax), GFP immunohistochemistry. (C) Normoxic embryo at 72 hpf; TCPT commissure (TCPTc) (arrow). (D) Embryo following hypoxia from 24–36 hpf; TCPTc is absent (arrow). (E) Graph showing percent Tg(foxP2-A.2:caax) embryos with wild-type TCPTc following hypoxia exposure at different ages. Error bars standard error of the proportion; ** p<0.0001. (F) C/L ratio determination. Confocal image of 72 hpf embryos illustrating determination of ratio of commissure intensity to intensity of longitudinal axons. (G) Quantification of TCPTc errors in Tg(foxP2-A.2:caax) embryos following hypoxia exposure at different ages. C/L (commissure:longitudinal) intensity ratio quantification along y-axis. *p<0.05; ** p<0.0001. ^mark indicates significant mortality at this stage. Errors bars standard error of the mean. (H) Normoxic embryo at 96 hpf; TCPT is intact (arrow). (I) Embryo at 96 hpf following 1% hypoxia from 24–36 hpf; TCPTc is absent (arrow). |
|
Hypoxia acts during development of the TCPTc by disrupting axon pathfinding. Confocal whole-mount images, anti-GFP immunohistochemistry, of Tg(foxP2-A.2:caax) embryos, ventral views, rostral top, except as noted images are maximum intensity projections, scale bars 50 μm except E–E2 and H2 25 μm; H3 12.5 μm. (A) Embryo at 24 hpf, cell bodies and initial axon processes are visible. (B) Embryo at 36 hpf; TCPTc has formed (arrowhead, magnified in inset). (C) TCPTc has formed completely by 72 hpf in normoxia. (D) Disruption of TCPTc in hypoxia. Dotted line shows the subsequent high-resolution pictures from the inset boxes: E–E3 from the left hand TCPTc with normal pathfinding; F–F3 with the disrupted TCPTc. (E) Higher magnification views of embryo at 72 hpf, single confocal slices, showing decussation of TCPT commissural and longitudinal axon tracts at different dorsal-ventral levels. (E2) Inset from dorsal-most level, single confocal slice, shows TCPTc arises as a single axon tract. (E3) Schematic of (E2) showing the single tract that will give rise to the TCPTc. (F) Mirror-images of the disrupted TCPTc from the right-hand of image (D), single confocal slices. The dorsal-most image shows the disrupted axon pathfinding. (F2) High-resolution single confocal slice, shows the two aberrant axon pathfinding errors made, 1 and 2, as axons leave the TCPT to join a longitudinal tract. (F3) Schematic of (F2) illustrating the aberrant pathfinding of axons leaving the TCPTc. (G) Hypoxic embryo at 96 hpf and higher resolution shown in (G2). (G3) is high-resolution ventral-to-dorsal single confocal slices. Arrows point to sparse axons giving rise to the TCPTc. Asterisks demonstrate axons aberrantly turning caudally and failing to join the TCPTc in the dorsal-most image. (G23) Schematic of (G3) illustrating the aberrant pathfinding of axons leaving the TCPTc at different ventral-dorsal levels. |
|
Developmental hypoxia does not affect general CNS development. Ventral views, rostral to the top, scale bar 50 μm (except E2, 25 μm). Whole-mount embryos, shown as brightfield (A), or confocal maximum intensity z-projections (B–E). (A, A2) in situ hybridization for dlx2 shows no difference in pattern. (B, B2) α-tyrosine hydroxylase (TH) antibody staining pattern is unaffected. (C, C2) Acridine orange shows similar numbers of apoptotic cells. (D, D2) Phospho-histone H3 antibody staining shows similar numbers of mitotic cells. (E) Confocal image of acridine orange stain demonstrating region for determining apoptotic cell counts. Inset shows high-magnification (E2) for counting cells in the 100 μm×100 μm area. |
|
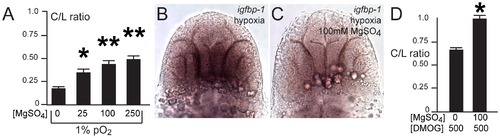
Developmental hypoxia induces the hif1 pathway and disrupts pathfinding via a non-cell autonomous mechanism. Whole-mount embryos, 36 hpf, ventral views, rostral to the top, scale bar 50 μm. Images are brightfield views. (A, B) Staining for the igfbp-1 gene activated by hypoxia shows that hypoxia effectively induces the hif1 pathway. (C) DMOG activates the hif1 pathway, as shown by increased igfbp-1 expression at 36 hpf following DMOG exposure from 24–36 hpf. (D) Increasing DMOG concentration from 24–36 hpf leads to increased rates of TCPTc pathfinding errors, assayed by C/L ratios. *p<0.05; ** p<0.005. Error bars SEM. (E–H) Confocal images, 72 hpf, ventral views, rostral top. (E, F) Normal TCPT pathfinding when hif1α or activated hif1αmut is expressed in foxP2 neurons. (G, H) Expression of hif1αmut, but not hif1α, disrupts TCPT pathfinding when expressed in neighboring neurons. (I, J) Expression of hif1αmut, but not hif1α, leads to activation of the hypoxia pathway as assayed by igfbp-1 expression. (K) C/L intensity ratios of experiments. *p<0.05; ** p<0.005. Error bars SEM. |
|
ephrinB2a mediates the hypoxia-induced TCPT pathfinding errors. (A–B) ephrinB2a is expressed in TCPT neurons. Confocal maximum intensity projections of whole-mount embryos, double immunohistochemistry for GFP and EfnB2a, ventral views, rostral top, scale bar 50 μm. (A–A3) TCPT neurons express ephrinB2a as they begin to extend axons. (B–B3) TCPT commissural axons express ephrinB2a as they cross the midline. (C–D) Hypoxia leads to increased expression of EfnB2a. Maximum intensity projections of 36 hpf embryos, ventral views, rostral to the top, EfnB2a immunohistochemistry. (E) Quantification of EfnB2a levels, normalized to normoxia. *p<0.05. Error bars SEM. (F) Knockdown of EfnB2a expression with morpholino rescues TCPTc pathfinding. Tg(foxP2-A.2:caax) embryo at 72 hpf; confocal maximum intensity projection, ventral view, rostral top, scale bar 50 μm. (G) C/L intensity ratio quantifications show ephrin morpholino rescue of hypoxia pathfinding errors. *p<0.01; ** p<0.001. Error bars SEM. (H) C/L intensity ratios show UAS:ephrinΔc rescues hypoxia pathfinding errors. ** p<0.001. Error bars SEM. |
|
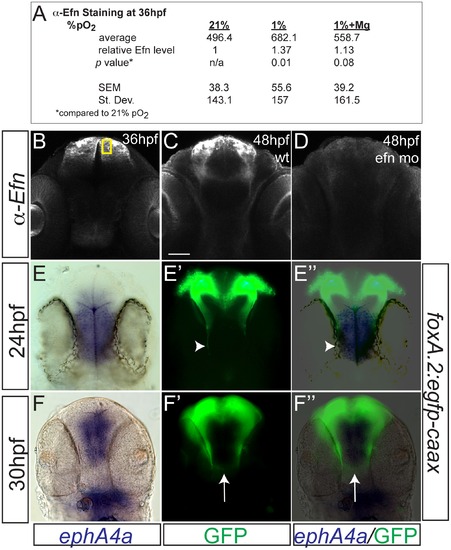
Hypoxia increased ephrinB2a expression in a pattern complementary to its receptor ephA4a. (A) Anti-EfnB2a intensity values at 36 hpf in the telencephalon, either following normoxia, or either hypoxia (1%), or hypoxia with 100 mM magnesium sulfate, from 24–36 hpf. (B) Confocal image of embryo at 36 hpf stained for ephrinB2, demonstrating the region of interest used to calculate α-ephrinB2 intensity. (C, D) Confocal images of embryos at 48 hpf stained for α-ephrinB2, demonstrating that efn morpholino (mo) effectively reduces ephrinB2a expression (D). (E–F3) Whole-mount double immunohistochemistry and in situs for GFP and ephA4a, respectively, in Tg(foxP2-A.2:caax) embryos. (E–E3) During initial axon extension at 24 hpf, axons (arrowhead) travel along the edge of ephA4a expression domain. (F–F3) At 30 hpf, as TCPTc forms (arrow), the axons avoid the area of ephA4a expression. |
|
Magnesium sulfate administration protects against hypoxia-related axon pathfinding defects. (A) C/L intensity ratios of increasing magnesium concentrations (mM). *p<0.01; ** p<0.001. Error bars SEM. (B, C) Magnesium reduces activation of the hif1α pathway, as shown by decreased igfbp-1 expression following hypoxia and concurrent magnesium exposure from 24–36 hpf. Bright-field images; ventral views, rostral top, scale bar 50 μm. (D) C/L intensity ratios shows rescue by magnesium of DMOG exposure from 24–36 hpf. *p<0.05. Error bars SEM. |