- Title
-
Zebrafish arl6ip1 Is Required for Neural Crest Development during Embryogenesis
- Authors
- Tu, C.T., Yang, T.C., Huang, H.Y., and Tsai, H.J.
- Source
- Full text @ PLoS One
|
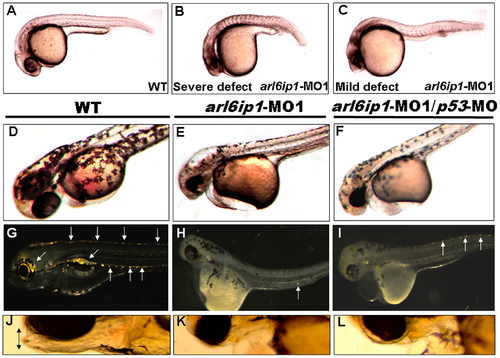
Embryos injected with arl6ip1-MO1 display abnormal development in ectoderm-derived tissues. Lateral views of embryos at 24 hpf (A-C), 48hpf (D-F) or at 96 hpf (G-L). (A) Wild-type (WT) embryos at 24 hpf displayed normal embryonic morphology. (B) Embryos injected with arl6ip1-MO1 exhibited foreshortened yolk extension and tail and small head with eye lesions. These embryos were defined as having severe defects. (C) Embryos with mild defects showed longer axis than embryos with severe defects. However, similar to embryos with severe defects, eye development was nearly equally compromised. (D) At 48hpf, WT embryos displayed plentiful melanophores. (E, F) Melanophores were highly reduced in arl6ip1-MO1 and arl6ip1-MO1/p53-MO-injected embryos. (G) Iridophores (arrows) were prominent in 4 dpf WT embryos. (H) Iridophores were almost absent in arl6ip1-MO1 embryos (only 1 residual cell was seen here in the ventral of the trunk) and (I) highly reduced in arl6ip1-MO1/p53-MO-injected embryos. (J) WT embryos showed well-developed head, jaw and melanophores. (K, L) arl6ip1-MO1 and arl6ip1-MO1/p53-MO-injected embryos displayed reduced head size and jaw (double arrows), but the head of arl6ip1-MO1/p53-MO embryos was bigger than arl6ip1 morphants. |
|
Craniofacial cartilage defects in the arl6ip1 morphants. Ventral views (A, C, D, H) or dorsal view (B) of 4-dpf embryos stained with Alcian blue to reveal craniofacial cartilage. Either pectoral fin (pf) or cartilage derived from mandibular (1st arch, m), hyoid (2nd arch, h), and ceratobranchial (3rd-7th arches, cb) arches was observed in control embryos (A). Dorsal views of anterior neurocranial cartilage in wild-type embryos showed paired trabeculae (tc) and an ethmoid plate (ep) (B). However, craniofacial cartilage and pectoral fin were absent in the arl6ip1-deficient (C) and arl6ip1-MO1/p53-MO-injected embryos (D). In the anterior neurocranial cartilage, trabeculae were severely reduced in both of these embryos, and the ethmoid plate was absent (C, D). dlx2 was expressed in migratory neural crest precursors of pharyngeal arch, as shown in dorsal views at 36 hpf in wild-type (E). Both arl6ip1-MO1-injected (F) and arl6ip1-MO1/p53-MO-injected embryos (G) had reduced dlx2 expression, and the reduction of dlx2 was prominent in posterior arches at 24 hpf. In addition, dlx2 was expressed normally in the forebrain (E, F, G). The severe chondrocranium and pectoral fin defects in arl6ip1-MO1/p53-MO-injected embryos were rescued by injection of 75 pg wobble arl6ip1 mRNA (H). bp, basal plate; pbc, posterior basic capsular commiss. Arrows, pectoral fin. |
|
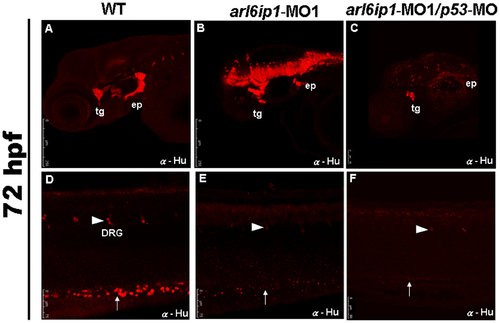
Neural crest derivatives are reduced in arl6ip1-deficient embryos. Lateral views (A-F) of 72 hpf embryos processed for anti-Hu immunofluorescence assaying (IFA) to reveal the derivatives of cranial neural crest cells by confocal micrographs. In control embryos (A, D), anti-Hu IFA-labeled trigeminal ganglia (tg), epibranchial ganglia (ep), dorsal root ganglia (drg) (arrowhead), enteric neurons (arrow) all appeared to be reduced either in arl6ip1 knockdown embryos (B, E) or arl6ip1-MO1/p53-MO-injected embryos (C, F). PHENOTYPE:
|
|
Expressions of ectodermal patterning factors appear normal in arl6ip1-MO1-injected embryos. Dorsal views of wild-type (A-E) and arl6ip1 morphant (F-J) embryos at the 3-somite stage. Anterior to the top. The neural ectodermal patterning factors of wnt8 (A, F), bmp4 (B, G), msxb (C, H), dlx3b (D, I), and pax3 (E, J). Normal expressions of these genes reveal that ectodermal patterning factors are not affected in arl6ip1 morphants, suggesting that Arl6ip1 does not have a role in ectodermal patterning. Scale bar: 100 μm. EXPRESSION / LABELING:
|
|
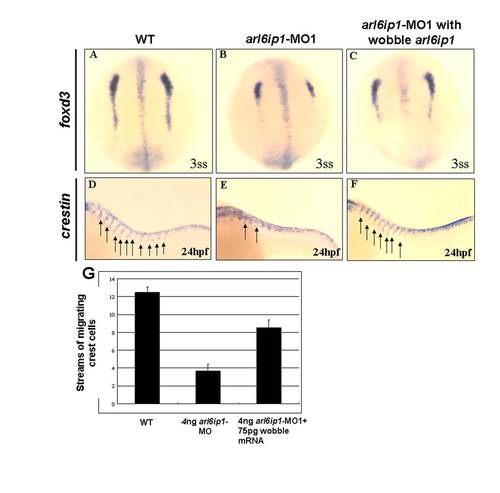
The markers of pre-migratory neural crest cells are down-regulated in arl6ip1-MO1-injected embryos. Dorsal views of 3-somite-stage wild-type (A, B, C, D) and arl6ip1 knockdown embryos (E, F, G, H), or 8-somite-stage wild-type (I, J, K,) and arl6ip1 knockdown embryos (L, M, N) were shown to reveal the indicated markers. Neural plate border was indicated with the expression of tfap2α (A, E), foxd3 (B, F), snai1b (C, G) and sox10 (D, H). Decreased expression of these markers, except tfap2α, was evident and predominant in arl6ip1 morphants. Arrows indicate position of midbrain-hindbrain boundary. (I-K) At the 8-somite stage, these markers were expressed predominantly in early migrating cranial neural crest cells (indicated by brackets). (L-N) These genes were reduced in embryos co-injected with 4 ng arl6ip1-MO1/6 ng p53-MO, especially in foxd3 and snai1b expression. EXPRESSION / LABELING:
PHENOTYPE:
|
|
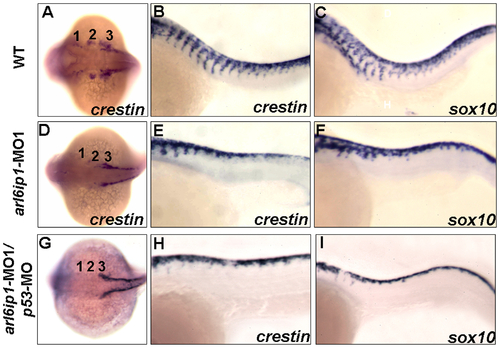
Abnormal cell migration in arl6ip1-MO1-injected embryos. Wild-type (A-C), arl6ip1-MO1-injected (D-F) and arl6ip1-MO1/p53-MO-injected (G-I) embryos were shown under dorsal views at 20-hpf (A, D, G) and under lateral views at 26-hpf (B, C, E, F, H, I), anterior to the left. Crestin expression revealed the migration of the three cranial neural crest streams in the wild-type embryos at 20 hpf (A). In arl6ip1 and arl6ip1/p53 knockdown embryos, crestin expression in the cranial crest was reduced in the 3rd neural crest stream and almost absent in the 1st and 2nd stream (D, G). At 26 hpf, the trunk migratory neural crest cells in wild-type embryos, as labeled by crestin and sox10, gradually migrated to ventral (B, C). However, the sox10- and crestin-expressing cells in arl6ip1 knockdown and arl6ip1-MO1/p53-MO-injected embryos failed to migrate ventrally (E, F, H, I). EXPRESSION / LABELING:
PHENOTYPE:
|
|
Enteric nervous system of arl6ip1 morphants is degenerated. Wild-type (WT) embryos (A, D, G), arl6ip1-MO1-injected embryos (B, E, H), and arl6ip1-MO1/p53-MO-injected embryos (C, F, I) were observed under dorsal view at 36-hpf (A-C) and under lateral view at 48 hpf (D, E, F) and 72 hpf (G, H, I). Neural crest cells (NCCs), which were labeled by crestin probe (arrowheads in A), of WT embryos exited the vagal region and migrated to the enteric region. However, the crestin-labeled NCCs of arl6ip1-MO1-injected and arl6ip1-MO1/p53-MO-injected embryos remained in the vagal region (B, C). Compared to WT embryos, either the arl6ip1-MO1-injected embryos or arl6ip1-MO1/p53-MO-injected embryos exhibited shortened enteric neurons and delayed migration (D vs. E; D vs. F; distance between two arrows). Additionally, the phox2b-positive enteric neurons were distributed throughout the entire gut to the anus (G). Arrows indicate the most posterior region of migration, and the asterisks represent the anus. The enteric neurons of arl6ip1-MO1-injected embryos and arl6ip1-MO1/p53-MO-injected embryos all failed to reach as far as the anus (H, I). EXPRESSION / LABELING:
PHENOTYPE:
|
|
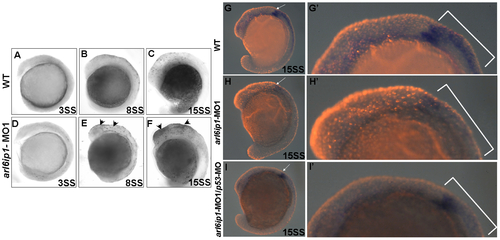
Cell death does not cause the loss of neural crest cells. Lateral views of TUNEL-labeled wild-type (WT) embryos (A-C) or arl6ip1-MO1-injected embryos (D-F) at 3-somite stage (A, D), 8-somite stage (B, E), and 15-somite stage (C, F), respectively. (A, D) There were no TUNEL-positive cells in WT and arl6ip1-MO1 embryos. (B, C, E, F) Compared to WT embryos, arl6ip-deficient embryos displayed a significant increase of TUNEL-positive cells in the dorsal region of embryos at the 8- and 15-somite stages (indicated by arrowheads) (B and C vs. E and F). (G, H, I) Double-labeling analysis with crestin (dark blue) and TUNEL (red fluorescent) showed that only limited cell death occurred at the expression region of crestin. (G′, H′, I′) The views of the embryos shown in panels G′, H′ and I′ were at higher magnification. PHENOTYPE:
|
|
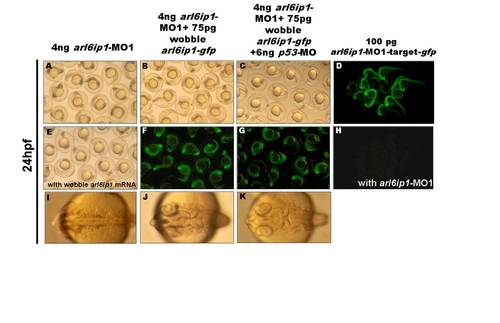
Confirmation of specific activities of arl6ip1-MO1 in zebrafish embryos. (A) Injection of 4 ng arl6ip1-MO1 caused brain lesions and foreshortened trunks. The defects of arl6ip1-MO1 were rescued either partially by the wobble arl6ip1 mRNA (E) and wobble arl6ip1-gfp mRNA (B) or almost completely by wobble arl6ip1-gfp mRNA with p-53 MO (C). (F, G) By detecting the GFP signals, we confirmed that the arl6ip1-MO1 cannot target wobble arl6ip1-gfp mRNA. (D) The Arl6ip1-GFP fusion protein was detected at 24 hpf in embryos injected with arl6ip1-MO1-target-gfp mRNA. (H) The GFP signal was absent at 24 hpf in embryos co-injected with arl6ip1-MO1-target-gfp mRNA and arl6ip1-MO1. (I) The arl6ip1 morphants did not display sulcus and gyrus in brains, and these brain defects were rescued either partially by wobble arl6ip1-gfp mRNA (J) or completely by wobble arl6ip1-gfp mRNA with p53-MO (K). |
|
The arl6ip1-MO1-injected embryos do not appear to have defective patterning in hindbrain. Wild-type (WT; A, C, E) and arl6ip1-knockdown (MO; B, D, F) embryos, either at 3-somite stage (3ss) (A-D) or at 24 hpf (E, F), were observed at dorsal view. (A-D) Neither fgf3 expression nor fgf8 expression in the arl6ip1 morphants was distinguishable from that of wild-type embryos (A vs. B; C vs. D). (E, F) Dorsal views of 24 hpf embryos processed for anti-Hu immunofluorescence staining (IFA) to reveal hindbrain segmentation. Similar to WT embryos, arl6ip1-knockdown embryos showed normal r1-r7 segmentation. fb, forebrain; mhb, midbrain-hindbrain border; pr4, premature rhombomere 4; r1–r7, rhombomere 1–7. |
|
Induction of neural crest cells occurs normally in arl6ip1-MO1 embryos. (A, B) Dorsal views of embryos at 10 hpf processed to show sox2 expression (labeling neural plate: np), and dlx3b expression (labeling the pre-placodal region: ppr). (A′, B′) Higher magnification views of right side of embryos shown in panels A and B, respectively. The region between these two expression domains was normally occupied by pre-migratory neural crest cells (indicated by brackets in A′ and B′). |
|
Injection of 75 pg wobble arl6ip1 mRNA enables embryos to rescue the defects induced by 4 ng arl6ip1-MO1. (A-C) Dorsal views of 3-somite-stage embryos, anterior to the top. (D-F) Lateral views of 24 hpf embryos, anterior to the left. (A, B) Compared to wild-type embryos, decreased expression of foxd3 was evident and predominant, especially in caudal region of pre-migratory neural crest cells in arl6ip1 morphants. (C) The down-regulation of foxd3 in arl6ip1-MO1 embryos was rescued by the wobble arl6ip1 mRNA. (D, E) Crestin-positive neural crest cells that normally migrate ventrally from the neural tube into the trunk were disrupted in arl6ip1 morphants (indicated by arrows). (F) Co-injection of wobble arl6ip1 mRNA with arl6ip1-MO1 recovered neural crest migration. (G) Quantification of the number of streams of sox10-labeled neural crest cells certified that migratory defects in arl6ip1 morphants can be rescued by wobble arl6ip1 mRNA. |
|
Abnormal Sonic Hedgehog signaling in arl6ip1-MO1/p53-MO morphants. (A-D) Lateral views of 24hpf embryos, anterior to the left. (A, B) Expression of shh was normal, either in wild-type embryos (A) or arl6ip1-MO1/p53-MO-injected embryos (B). (C, D) pax6, a gene negatively regulated by Shh signaling, was up-regulated in the neural tube of arl6ip1-MO1/p53-MO-injected embryos (D). Neural tube: indicated by arrow. PHENOTYPE:
|
|
Knockdown of Arl6ip1 induces KV cilia defects. (A-D) pitx2 normal expression in left lateral plate mesoderm (LPM)(arrow) at 20-somite stage was disrupted in embryos injected with arl6ip1-MO1/p53-MO. (E-H) At 20-somite stage, spaw is expressed in the left LPM (arrow), while knockdown of Arl6ip1/P53 resulted in left (E), absent (F), bilateral (G) or rightward (H) spaw expression in the LPM. (I, J) Confocal microscopic images of anti-acetylated tubulin staining of KV cilia (red fluorescent) at 8-somite stage. In the wild-type embryos, cilia can be observed in a spherical pattern in the region of KV (I). Cilia number, length and KV area reduced in arl6ip1-MO1/p53-MO morphants at 8-somite stage (J). (K) Summary of pitx2 and spaw asymmetrical gene expression patterns. PHENOTYPE:
|
|
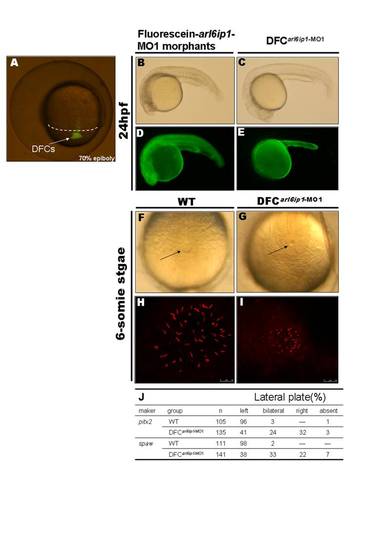
Specific knockdown of Arl6ip1 in DFCs alters LR development without other effects on embryogenesis. (A) As observed under fluorescent microscopy, injection of fluorescein-tagged arl6ip1-MO1 into mid-blastula stage embryos was observed in dorsal forerunner cells (DFCs) 3–4 hr post-injection at 70% epiboly stage. The dorsal margin was indicated by dashed line. (B–E) Lateral views of 24hpf embryos. Embryos were observed under transmitted light microscopy (B, C) or fluorescent microscopy (D, E). MOs were present in all cells of embryos injected at the one-cell stage (D), and severe defects induced by fluorescein-tagged arl6ip1-MO1 embryos were shown (B). Fluorescein-tagged arl6ip1-MO1 injected into embryos at mid-blastula stage was primarily found in the yolk cell and yolk tube (E), indicating that cells, other than DFC and yolk, did not incorporate MO. These DFC of arl6ip1-MO1-injected embryos (DFCarl6ip1-MO1) developed a normal morphology (C) similar to WT embryos. (F, G) Tail views of 6-somite stage embryos. KV was indicated by arrows. Compared to WT embryos (F), DFCarl6ip1-MO1 embryos showed reduced KV at 6-somite stage (G). (H, I) Confocal microscopy images of anti-acetylated tubulin staining of KV cilia (red fluorescent) at 6-somite stage. Cilia distributed over a spherical pattern in the region of KV (H). However, cilia were disorganized in reduced KV of DFCarl6ip1-MO1 embryos (I). (K) Summary of pitx2 and spaw asymmetrical gene expression patterns. |

Unillustrated author statements |