- Title
-
At the Start of the Sarcomere: A Previously Unrecognized Role for Myosin Chaperones and Associated Proteins during Early Myofibrillogenesis
- Authors
- Myhre, J.L., and Pilgrim, D.B.
- Source
- Full text @ Biochem. Res. Int.
|
Summary of steps involved in myocyte differentiation and myofibrillogenesis, with regards to actin dynamics. (a) Proliferating myoblasts are derived from determined myotome cells and possess an unspecialized actin cytoskeleton (grey lines). (b) As differentiation begins, these cells aggregate, characterized by the formation of localized stress fibers in a cortical actin wall (insert). Contractile function of these fibers is provided by nonmuscle myosin (NMM, arrow). (c) Myoblasts align themselves concurrent to substrate attachment and the elaboration of protocostameres (arrow). The stress-fiber-like cortical actin and NMM will form the premyofibril templates for subsequent myofibril assembly. (d) Fusion occurs, resulting in the formation of multinucleated myotubes. Myofibrils begin to form at the cell periphery, centered on costamere attachment points (arrow), constructed from premyofibril templates (insert). Premyofibrils consist of alternating bands of membrane-associated |
|
Schematic diagram of the sarcomere and costamere protein complexes of striated muscle cells. Major components of the mature sarcomere and costamere are shown, along with the cytoskeletal and motor filament systems, in context with the sarcolemma and organelles of syncytial myocytes. Known chaperone or cochaperone molecules are shown in bold, along with their substrates. Arrows indicate regions where chaperone-mediated protein folding is essential to incorporate polymeric filament proteins. Modified from Sparrow and Schock [ |
|
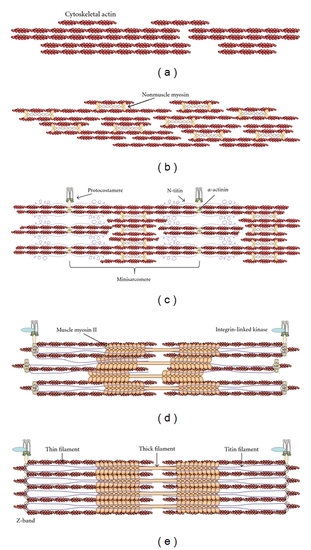
Synthesis of the premyofibril model with the roles of nonmuscle myosin (NMM) in early differentiating myoblasts. Schematic representation of the molecular events leading from cytoskeletal actin to mature myofibrils. (a) Elaboration of the actin cytoskeleton in proliferating myoblasts leads to the formation of a cortical actin wall. (b) Stress-fiber-like structures in the cortical actin wall contain associated NMM-II, which allows for alignment and fusion of myoblasts. (c) Alignment and fusion are concurrent with early costamere formation, resulting in the anchorage of premyofibrils to the extracellular matrix. These sites serve as nucleation points, resulting in the formation of minisarcomeres with alternating bands of |
|
Proposed model of roles for Unc45b/nonmuscle myosin during early myofibrillogenesis. (a) Merged protein model of Unc-45b from the X-ray crystal structure of |