- Title
-
Visualizing digestive organ morphology and function using differential fatty acid metabolism in live zebrafish
- Authors
- Carten, J.D., Bradford, M.K., and Farber, S.
- Source
- Full text @ Dev. Biol.
|
BODIPY-FL C16 accumulates in lipid droplets in the digestive organs of live larval zebrafish. In all images, anterior is to the right to allow liver viewing. A. Enterocytes of the anterior intestinal bulb readily absorb BODIPY-FL C16 following an egg yolk and analog feed. The dotted box indicates a row of intestinal enterocytes exhibiting apical and basolateral accumulation of BODIPY-FL C16. Larger gaps in intestinal fluorescence (arrow) are likely non-absorbing enteroendocrine cells (Wallace et al., 2005). B. A schematic of lipid metabolism in polarized intestinal enterocytes. Enterocytes take up lipids from the intestinal lumen, form lipid droplets (ld) and secrete basolateral chylomicrons (cm). The basement membrane (bm) and cell nucleus (n) are also indicated. C. Intestinal enterocytes (dotted box indicates a single enterocyte) of larvae fed BODIPY-FL C16 display apical lipid droplets and smaller basolateral fluorescent foci reminiscent of chylomicrons. The intestinal lumen is indicated (asterisk). D. BODIPY-FL C16 accumulates within large hepatic lipid droplets (filled arrowhead) and faintly in hepatic ducts (arrow). Hepatocyte nuclei (empty arrowhead) are also visible. E. BODIPY-FL C16 accumulates throughout the exocrine pancreas, forming small fluorescent foci (arrowhead). Beneath the exocrine pancreas, an intestinal blood vessel (arrow) running the length of the intestine fluoresces, indicating analog entry into the vascular system. A is a composite of 3 separate confocal images. Scale bars = 10μm. (n = 3; 6 larvae per feed.) |
|
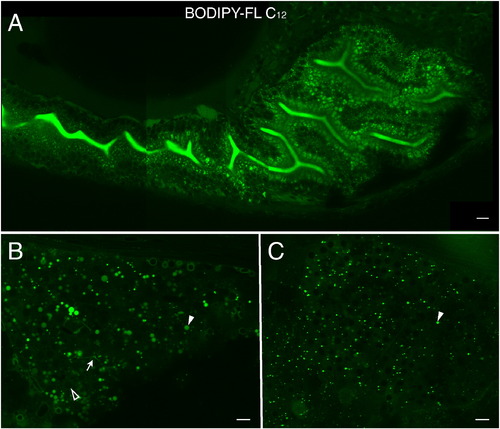
BODIPY-FL C12 is absorbed and transported in the digestive organs of zebrafish larvae. A. The analog appears in the intestinal lumen as well as in lipid droplets in intestinal enterocytes. Diffuse cellular accumulation of BODIPY-FL C12 is consistent with the analog′s shorter chain length, decreased hydrophobicity, faster metabolism, and entry into more subcellular compartments. B. BODIPY-FL C12 readily accumulates in the liver, visualizing large lipid droplets (filled arrowhead), hepatic ducts (arrow), and hepatocyte nuclei (empty arrowhead). C. BODIPY-FL C12 accumulates in fluorescent foci (arrowhead) in the exocrine pancreas. A Scale bars = 10μm. (n = 3; 6 larvae per feed.) |
|
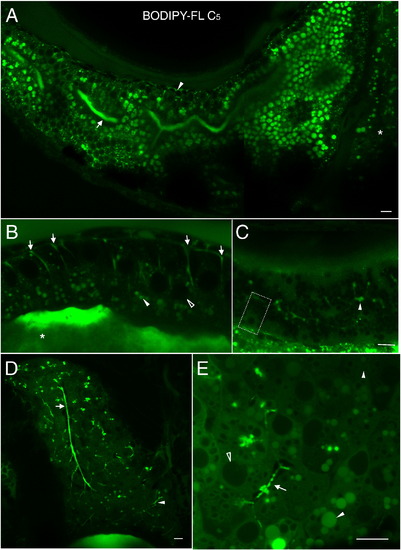
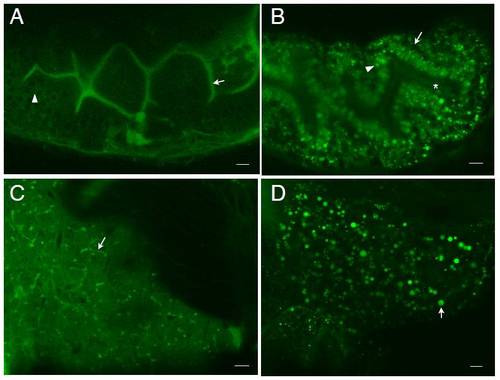
BODIPY-FL C5 reveals significant subcellular details within larval digestive organs (6dpf). A. In enterocytes of the anterior intestine BODIPY-FL C5 appears in structures reminiscent of lipid droplets. Lumenal accumulation of BODIPY-FL C5 in the intestine (arrow) is consistent with the expedited metabolism, transport and biliary concentration of medium chain fatty acids. The analog also appears in a dorsal intestinal blood vessel (arrowhead) and in the liver (asterisk). B. Subcellular enterocyte morphology is visualized following a BODIPY-FL C5 feed. Lipid droplets (filled arrowhead), a nucleus (empty arrowhead), apparent pericellular accumulation of the analog (arrows) and the intestinal lumen (asterisk) are indicated. C. BODIPY-FL C5 visualizes subcellular details of acinar cells and pancreatic ducts in the larval exocrine pancreas. A single acinar cell (dashed box) containing a basal nucleus and apical zymogen granules, as well as pancreatic ducts (arrowhead) is indicated. D. BODIPY-FL C5 feeding illuminates the intrahepatobiliary ductal network. In the right liver lobe, a single long duct (arrow), numerous interconnecting ducts and terminal ductules (arrowhead) are illuminated (see also Supplementary Movie-1). E. Subcellular details of larval hepatocytes are revealed by BODIPY-FL C5 feeding. Lipid droplets (filled arrowhead), hepatocyte nuclei (empty arrowhead), and intrahepatic ducts (arrow) are indicated. A. is a composite of 2 separate confocal images. Scale bars = 10μm. (n = 3; 6 larvae per feed.) BODIPY-FL C5 reveals significant subcellular details within larval digestive organs (6dpf). A. In enterocytes of the anterior intestine BODIPY-FL C5 appears in structures reminiscent of lipid droplets as well as in small endosomal-like compartments. Lumenal accumulation of BODIPY-FL C5 in the intestine (arrow) is consistent with the expedited metabolism, transport and biliary concentration of medium chain fatty acids. The analog also appears in a dorsal intestinal blood vessel (arrowhead) and in the liver (asterisk). B. Subcellular enterocyte morphology is visualized following a BODIPY-FL C5 feed. Lipid droplets (filled arrowhead), a nucleus (empty arrowhead), apparent pericellular accumulation of the analog (arrows) and the intestinal lumen (asterisk) are indicated. C. BODIPY-FL C5 visualizes subcellular details of acinar cells and pancreatic ducts in the larval exocrine pancreas. A single acinar cell (dashed box) containing a basal nucleus and apical zymogen granules, as well as pancreatic ducts (arrowhead) indicated. D. BODIPY-FL C5 feeding illuminates the intrahepatobiliary ductal network. In the right liver lobe, a single long duct (arrow), numerous interconnecting ducts and terminal ductules (arrowhead) are illuminated (see also Supplementary Movie-1). E. Subcellular details of larval hepatocytes are revealed by BODIPY-FL C5 feeding. Lipid droplets (filled arrowhead), hepatocyte nuclei (empty arrowhead), and intrahepatic ducts (arrow) are indicated. A. is a composite of 2 separate confocal images. Scale bars = 10μm. (n = 3; 6 larvae per feed.) |
|
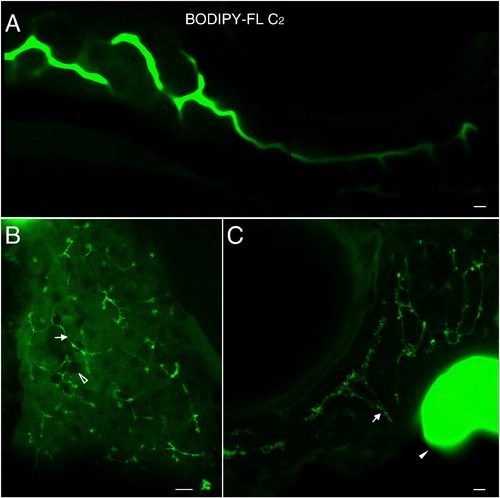
BODIPY-FL C2 does not participate appreciably in lipid metabolism. A. Unlike the longer chain length analogs, BODIPY-FL C2 fluorescence appears primarily in the intestinal lumen of larvae. B. In the liver BODIPY-FL C2 accumulates in the hepatic ducts (arrow) and diffusely in hepatocytes, whose nuclei are discernable (empty arrowhead). C. The ductal network of the exocrine pancreas is also labeled (arrow). The gall bladder (filled arrowhead) is indicated in C. A. is a composite of 2 separate confocal images. Scale bars = 10μm. (n = 3; 6 larvae per feed). |
|
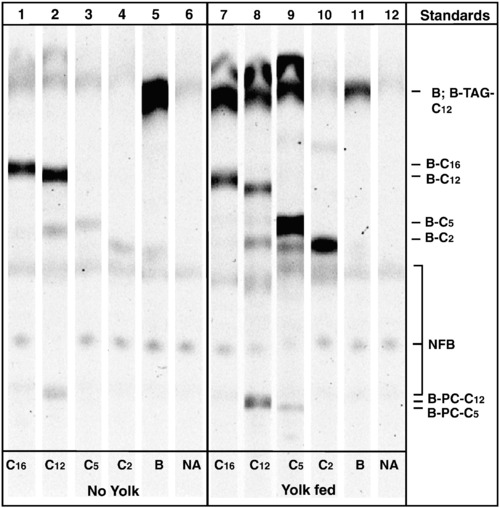
Egg yolk feeding enhances the uptake and metabolism of BODIPY fatty acid analogs by zebrafish larvae. Thin layer chromatography analysis of total larval lipids extracted following analog feeds in the absence (lanes 1–5) and presence (lanes 7–11) of egg yolk. Lanes 6 and 12 are no analog (NA) unfed and yolk fed controls, respectively, and exhibit faint naturally fluorescent lipid bands (NFB). A polar solvent system was used to resolve fluorescent phospholipids (bottom of plate), free analogs (middle), and neutral lipids (top of plate). Abbreviations for fluorescent lipid standards are as follows: BODIPY-Cholesteryl (B-CE-C12), BODIPY-Triacylglyceride (B-TAG-C12), BODIPY493/503 (B), BODIPY-C16 (B-C16), and BODIPY-Phosphatidylcholine (B-PC-C12, B-PC-C5). |
|
Medium and long chain fatty acid BODIPY analogs are synthesized into triacylglyceride. A second protonating solvent system was used to separate BODIPY493/503 (B) from BODIPY-Triacylglyceride (B-TAGs). Lipid extracts from larvae fed egg yolk without analog (NA) appear in lane 6. (n = 3 concurrent feeds). |
|
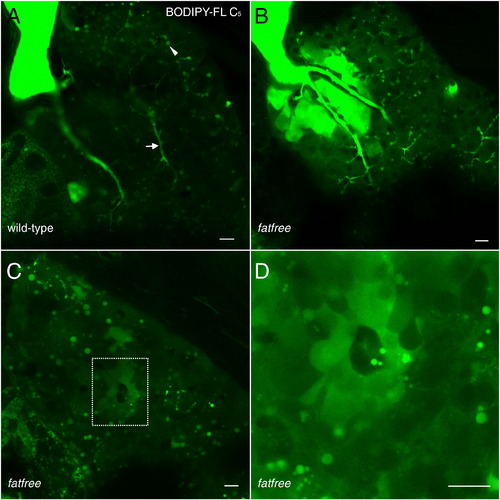
Fat-free (ffr) larvae display abnormal bile accumulation upon BODIPY-FL C5 feeding. A. Wildtype siblings (controls) from a cross of ffr +/- larvae (6dpf) exhibit normal hepatic accumulation of BODIPY-FL C5 in lipid droplets (filled arrowhead) and hepatic ducts (arrow). B. Hepatic ducts in ffr -/- larvae leak or abnormally accumulate BODIPY-FL C5, consistent with the hepatobiliary structural malformations previously identified in these mutants. C. Analog labeling also reveals ffr -/- hepatocytes are unable to secrete bile as indicated by the appearance of cholestatic livers. D. A higher magnification of C. reveals abnormal accumulation of bile in a single ffr -/- hepatocyte. Scale bars = 10μm. (n = 3 feeds; 10 larvae per feed). PHENOTYPE:
|
|
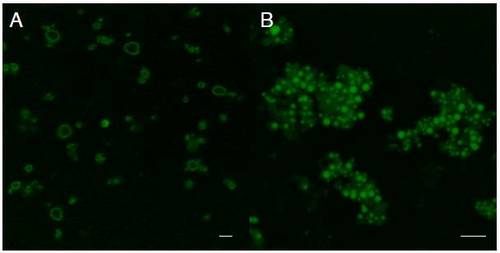
Fluorescent liposomes are formed following addition of BODIPY-FL analogs to sonicated egg yolk. A. BODIPY-FL C16 partitions preferentially into liposomal membranes making liposomes appear as fluorescent rings when viewed using confocal microscopy. B. BODIPY-FL C5 partitions into the liposomal core, making liposomes appear as fluorescent circles. BODIPY-FL C12, C2, and the BODIPY alone also partition into the aqueous core of liposomes (data not shown). Scale bars are 5 μm. |
|
The BODIPY fluorophore enters intestinal but not hepatic lipid droplets. A. BODIPY-fed larvae (6 dpf) exhibit fluorescent intestinal lipid droplets (arrow) throughout the enterocytes of their anterior intestine. B. In the liver, BODIPY appears mainly in the hepatic ducts (arrow) and diffusely in the cytoplasm of hepatocytes. Hepatocyte nuclei are indicated (arrowhead). Scale bars = 10μm (n = 3 feeds; 4 larvae imaged per feed). |
|
Egg yolk feeding enhances BODIPY-FL C16 uptake and lipid droplet formation in the larval intestine and liver. A. Zebrafish larvae (6 dpf) fed BODIPY-FL C16 in the absence of egg yolk exhibit fluorescence in the intestinal lumen (arrow) and faintly in enterocytes (arrowhead). B. Co-feeding BODIPY-FL C16 with egg yolk enhances analog uptake by intestinal enterocytes. Lipid droplets (arrowhead) appear in enterocytes surrounding the intestinal lumen (asterisk). Nuclei (arrow) appear as dark circles. C. Larvae fed BODIPY-FL C16 in the absence of egg yolk have diffuse accumulation of the analog in their livers. Few lipid droplets appear in hepatocytes, with fluorescence appearing mainly in the cytoplasm of hepatocytes and in hepatic ducts (arrow). D. The livers of larvae co-fed BODIPY-FL C16 with egg yolk have lipid droplets (arrow). Scale bars = 10μm (n = 3 feeds; 4 larvae imaged per feed.) |
Reprinted from Developmental Biology, 360(2), Carten, J.D., Bradford, M.K., and Farber, S., Visualizing digestive organ morphology and function using differential fatty acid metabolism in live zebrafish, 276-85, Copyright (2011) with permission from Elsevier. Full text @ Dev. Biol.