- Title
-
Zebrafish foxo3b Negatively Regulates Canonical Wnt Signaling to Affect Early Embryogenesis
- Authors
- Xie, X.W., Liu, J.X., Hu, B., and Xiao, W.
- Source
- Full text @ PLoS One
|
Sequence comparison of zebrafish foxo3b with other FOXOs and developmental expressing patterns of zebrafish foxo3b. (A) Sequence alignment of zebrafish foxo3b and human FOXO3a protein. (B) Neighbor-Joining Analysis of vertebrate FOXO protein sequences. Phylogenetic analysis was conducted using MEGA version 5 (Tamura, Peterson, Stecher, Nei, and Kumar 2011). hFoxo3a (Homo sapiens, Accession number NM_001455), hFoxo6 (XM_002342102), hFoxo4 (NM_005938), hFoxo1 (NM_002015), mFoxo3 (Mus musculus, NM_019740), mFoxo6 (NM_194060), mFoxo4 (NM_018789), mFoxo1 (NM_019739), raFoxo3 (Rattus norvegicus, NM_001106395), raFoxo6 (XM_001057233), raFoxo4 (NM_001106943), raFoxo1 (NM_001191846), zFoxo3b (Danio rerio, NM_131085), zFoxo3a (NM_001009988), zFoxo1a (NM_001077257), zFoxo1b (NM_001082857), xeFoxo3 (Xenopus laevis, NM_001092949), xeFoxo6 (NM_001159282), xeFoxo4 (FJ811896), xeFoxo1a (NM_001092948), xifoxo5 (Xiphophorus maculates, AY040320). NJ bootstrap values were shown on the branches. (C) The expression pattern of zebrafish foxo3b during embryogenesis. (C1–C3) The expression of foxo3b was detected at 2-cell stage embryos, and became weaker at oblong stage. (C4, C5) Foxo3b was ubiquitously expressed at 40% epiboly stage, a stronger expression was observed at shield stage. (C6, C7) Foxo3b became weaker at bud stage; at 6-somite stage, its expression level almost recovered to that of shield stage embryos. (C8, C13) Foxo3b was observed in the developing eye, hindbrain and posterior mesoderm by 24 hpf. (C9, C10) By 55 hpf, foxo3b expression was confined to the anterior central nervous system (CNS), with weak expression in the heart. (C11, C12, C14) By 72 hpf, foxo3b expression became weaker, but continued in the CNS and heart. C1–C4, lateral view; C5, lateral view with dorsal to the right; C6, C7, lateral view with anterior on top; C8, C9, C11, C13, C14, lateral views with anterior to the left; C10, C12, dorsal views with anterior on top; r, retina; p, pectoral fin bud; hb, hindbrain; pm, posterior mesoderm; pd, pronephric duct; h, heart; t, telencephalon; ov, otic vesicle; m, mesencephalon; c, cell; s, somite; h, hours post-fertilization (hpf). (D) Relative RNA expression levels as determined by semi-quantitative RT-PCR. For each stage, oligo dT-primed cDNA was used as template for three separate PCR amplifications using primers for foxo3b and 18s (internal control), foxo3b reached a high expression level at shield stage. For quantitative purpose, mRNA expression levels were normalized to 6 hpf (1.00). EXPRESSION / LABELING:
|
|
Knockdown of foxo3b results in defects in body axis and brain. (A) Validation of foxo3b ATG-blocking morpholino (foxo3b-ATG-MO). A1, embryos were injected with STD-MO (8 ng per embryo, control) and a wild-type foxo3b-GFP fusion protein expression vector (WT) and then examined by fluorescence microscopy; A2, embryos were injected with foxo3b-ATG-MO (8 ng per embryo) and a wild-type foxo3b-GFP fusion protein expression vector (WT); A3, embryos were injected with STD-MO and a mutated foxo3b-GFP fusion protein expression vector (MT); A4, embryos were injected with foxo3b-ATG-MO and a mutated foxo3b-GFP fusion protein expression vector (MT). A1-A4, bud stage. (B, C) Morphology of representative morphants in foxo3b-ATG-MO injected embryos. The morphants had shorter body length, abnormal brain and heart at 50 hpf. Black box, dead embryos at 24 hpf; B3, B8, B9, dark gray box, embryos with defects at 50 hpf characterized by severe phenotype: no blood circulation, severely reduced body length and thinner brain; B2, B6, B7, light gray box, embryos with mild phenotype; B1, B4, B5, white box, un-injected wild-type embryos. B1-B3, front views; B4, B6, B8, lateral views; B5, B7, B9, lateral views with anterior to the left. (D) Validation of foxo3b splice-blocking morpholino (foxo3b-SP-MO). D1, Foxo3b exon/intron structure. Foxo3b-SP-MO can alter splicing of foxo3b mRNA, which results in the production of an aberrantly spliced message (as showed by red line). D2, The injection of foxo3b-SP-MO results in the production of a truncated mRNA (440 bp). The embryos were collected at bud stage, and β-actin was used as an internal control. (E, F) Morphology of splice-MO injected embryos. By 55 hpf, the morphants showed defects similar to that of foxo3b-ATG-MO injected embryos. (G) The expression level of foxo3a was not altered in foxo3b-knockdown or foxo3b over-expressed embryos. Embryos were injected with 8 ng foxo3b-ATG-MO (G2) or 1 ng foxo3b mRNA (G3) at 1-cell stage. Wild-type embryos were used as control. G1-G3, bud stage, lateral views. PHENOTYPE:
|
|
Loss of foxo3b function results in anterior defects. (A) The foxo3b-ATG-MO injected embryos showed remarkable loss of expression of anterior neural markers by 24 hpf, which could partially be rescued by co-injection of foxo3b mismatch mRNA. (A1–A3, A13) The expression of nkx5.1 at the telencephalon (indicated by white arrows) was dramatically reduced in foxo3b-ATG-MO injected embryos. Co-injection of foxo3b mismatch mRNA could partially restore its expression at the telencephalon. (A4-A6, A14) Six3b was specifically expressed at the telencephalon and eyes. Loss of foxo3b function resulted in abnormal expression pattern of six3b, which was restored by co-injection of foxo3b mismatch mRNA. (A7–A9, A15) Pax6 expression at the forebrain and eyes decreased greatly in foxo3b morphants. Co-injection of foxo3b mismatch mRNA partially restored its expression. (A10–A12, A16) Expression of tbx5 at the retina was dramatically reduced in foxo3b-knockdown embryos. Its expression was rescued by co-injection of foxo3b mismatch mRNA. (A17) Opl expression at the telencephalon was reduced in foxo3b morphants compared to control embryos, which was efficiently rescued by co-injection of foxo3b mismatch mRNA. Embryos were injected with 8 ng foxo3b-ATG-MO or 125 pg foxo3b mismatch mRNA,wild-type embryos were used as control. A1-A3, lateral views with anterior to the left; A4-A12, dorsal views with anterior to the left; A1-A17, 24 hpf. (B) The foxo3b-SP-MO injected embryos exhibited anterior defects similar to that of foxo3b-ATG-MO injected embryos. (B1–B2, B7) The expression of pax6 at the telencephalon and eyes was reduced in foxo3b-SP-MO injected embryos. (B3–B4, B8) Tbx5 expression at the eyes decreased in foxo3b-knockdown embryos. (B5–B6, B9) Loss of zygotic foxo3b function resulted in reduction of nkx5.1 expression at the telencephalon. Embryos were injected with 16 ng STD-MO (control) or 16 ng foxo3b-splice-MO. B1–B4, dorsal views with anterior to the left; B5-B6, lateral views with anterior to the left; B1-B9, 24 hpf. |
|
Knockdown of foxo3b leads to defects in DV patterning during early embryogenesis. (A) A1–A4, sqt expression increased in foxo3b-MO injected embryos compared to control embryos. A5–A8, presumptive organizer marker flh expanded at foxo3b-knockdown embryos. A9–A10, foxo3b knockdown caused ectopic vox expression at 30% epiboly. (B) The expression of presumptive organizer marker gsc and flh in foxo3b-MO injected embryos. The arrows identified embryos with expanded expression (B2, 74%, n = 23; B4, 52%,n = 21, respectively) and asterisks identified embryos with decreased expression. Embryos were injected with 8 ng STD-MO (control) or 8 ng foxo3b-ATG-MO. A1–A4, lateral views; A5–A12, B1–B4, animal pole views; A1–A12, 30% epiboly; B1–B4, shield stage. EXPRESSION / LABELING:
PHENOTYPE:
|
|
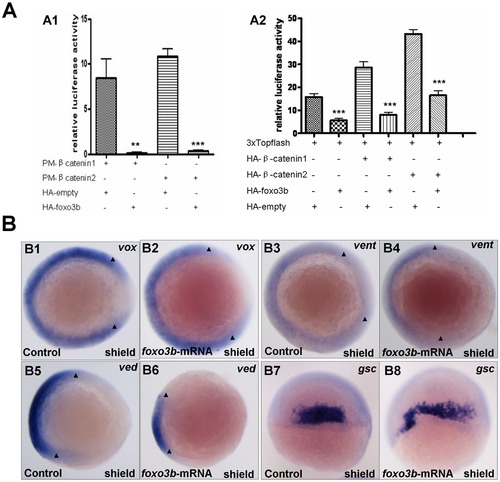
Foxo3b gain-of-function inhibits Wnt/β-catenin signaling in embryos. (A) Foxo3b inhibited β-catenin/T cell factor activity in embryos. A1, 1-cell stage embryos were injected with a mixture of plasmids as indicated, together with pFR-luc as a reporter gene and pTK-renilla as an internal control; luciferase activity was measured after 11h. Date presented are the average (±SEM) of four independent experiments. A2, 1-cell stage embryos were injected with 3xTOPFlash, and the plasmids as indicated, together with pTK-renilla as an internal control; luciferase activity was measured after 11 h, performed in triplicate. "**" indicates p<0.01; "***" indicates p<0.001. (B) Gain-of-function of foxo3b resulted in suppression of Wnt/β-catenin signaling in embryos. B1-B2, foxo3b over-expressed embryos showed reduced vox expression (arrowheads in B1 and B2) compared to wild-type. B3-B6, the expression of ventral marker vent and ved (domain width indicated by arrowheads) decreased in 70% (n = 20) and 75% (n = 16) of foxo3b-ATG-MO-injected embryos respectively. B7-B10, the expression domain of dorsal marker gsc expanded in most foxo3b over-expressed embryos. Embryos were injected with 2 ng GFP mRNA (control) or 2 ng foxo3b mRNA. B1-B6, animal pole views with dorsal to the right; B7-B8, dorsal views with anterior on top; B1-B8, shield stage. |
|
Foxo3b functions in posterior neuroectoderm formation and involves with BMP signaling in DV patterning. (A) Foxo3b affected posterior neuroectoderm formation and mesoderm induction. A1-A2, at 80% epiboly, cdx4 expression expanded in foxo3b-MO injected embryos compared to control embryos. A3-A4, the expression of non-neural ectodermal marker gata2 was up-regulated in foxo3b-knockdown embryos. A5-A6, ectoderm marker foxi1 expanded in foxo3b morphants. (B) The expression of wnt8 and bmp ligands in foxo3b morphants. B1–B2, wnt8 expression increased in foxo3b morphants at shield stage. B3–B6, the expression of ventral markers bmp2b/bmp4 was up-regulated in foxo3b-knockdown embryos at shield stage. Embryos were injected with 8 ng STD-MO (control) or 8 ng foxo3b-ATG-MO. A1–A2, lateral views with dorsal to the right; A3–A6, animal pole views with dorsal to the right; B1–B6, lateral views with dorsal to the right; A1–A2, 80% epiboly; A3–A6, B1–B6, shield stage. |
|
Foxo3b inhibits both maternal and zygotic Wnt/β-catenin signaling. (A) Foxo3b morphants could be rescued by knockdown of β-catenin1. Black box, dead embryos at 24 hpf; A5-A6, dark gray box, embryos with defects at 55 hpf characterized by severe phenotype (as described in Fig. 2B); A3-A4, light gray box, embryos with mild phenotype; A1–A2, white box, un-injected wild-type embryos. A1, A3, A5, lateral views; A2, A4, A6, lateral views with anterior to the left; A1–A7, 55 hpf. (B) Anterior defects caused by loss of foxo3b function could be rescued by co-injection of dnTCF mRNA. (B1–B3, B7) Six3b was expressed abnormally in foxo3b morphants, which could be rescued by co-injection of dnTCF mRNA by 24 hpf. (B4–B6, B8) Tbx5 expression at the eyes was greatly reduced in foxo3b-knockdown embryos. Co-injection of dnTCF mRNA could efficiently restore its expression at the eyes. (B9–B11) Nkx5.1, pax6 and opl expression at the anterior neural plate could also be efficiently rescued by co-injection of dnTCF mRNA. Embryos were injected with 8 ng foxo3b-ATG-MO or 10 pg dnTCF mRNA,wild-type embryos were used as control. B1–B6, dorsal views with anterior to the left; B1–B11, 24 hpf. (C) Foxo3b knockdown resulted in abnormal expression of early Wnt target genes, which could be rescued by co-injection of β-catenin1/2 morpholino. (C1–C8, C15) The morphants showed increased expression of gsc, which could also be rescued by co-injection of β-catenin1 MO. Co-injection of β-catenin2 MO resulted in dramatically reduction of gsc expression (75%, n = 32) compared to control embryos. (C9-C14, C16) The expression level of vox increased dramatically in foxo3b-knockdown embryos, which could be partially rescued by co-injection of β-catenin2 MO. (C17) At 30% epiboly, sqt expression was up-regulated in foxo3b morphants. Co-injection of β-catenin1 morpholino efficiently reduced its expression. Embryos were injected with 8 ng foxo3b-ATG-MO or 8 ng β-catenin1/2 morpholino,wild-type embryos were used as control. C1-C14, dorsal views; C1-C17, 30% epiboly. PHENOTYPE:
|
|
Foxo3b interacts with β-catenin1/2 in 293T cell. (A) Foxo3b inhibited β-catenin/T cell factor activity in 293T cell line. 293T cells were transfected with 3xTOPFlash, and the plasmids as indicated, together with pTK-renilla as an internal control; luciferase activity was measured after 24h. Date presented were the average (±SEM) of three independent experiments, performed in triplicate. “**” indicates p<0.01. (B) Foxo3b co-localized with β-catenin1/2. HeLa cells were transfected with GFP-β-catenin1 (B1-B3) or GFP-β-catenin2 (B4–B6), together with RFP-foxo3b. Transfected cells were then observed using fluorescent microscopy after 24h. (C) Foxo3b interacted with β-catenin1/2. 293T cells were transfected with the indicated expressing plasmids. HA-foxo3b was immunoprecipitated, and binding of Flag-β-catenin1 (C1) or Flag-β-catenin2 (C2) was analyzed by immunoblotting. |
|
Bmp2b expression was rescued in foxo3b-knockdown embryos co-injected with foxo3b mismatch mRNA. Animal views, shield stage. |