- Title
-
Plasticity of photoreceptor-generating retinal progenitors revealed by prolonged retinoic acid exposure
- Authors
- Stevens, C.B., Cameron, D.A., and Stenkamp, D.L.
- Source
- Full text @ BMC Dev. Biol.
|
Opsin expression is altered by retinoic acid treatment beginning at the time of retinal neurogenesis. Embryos were treated with DMSO (Cont.; A, B, C), or 0.3 μM RA (D, E, F) at 36 hpf, and at 60 hpf were hybridized as whole mounts with probes corresponding to rod opsin (A, D), red cone opsin (B, E), or blue cone opsin (C, F); views are of whole embryonic eyes; dorsal is up and nasal to the left. In the control (DMSO-treated) embryos, rod photoreceptors are found predominantly in the ventral and dorsal regions (A), while red and blue cones (B, C) are evenly spread across the retina. In RA-treated embryos there is an apparent increase in rods, particularly in central regions of the retina (B), and a decrease in the appearance of red cones, leading to empty patches in the red cone mosaic (E, arrows). Boxed regions in each panel appear at higher magnification in the insets. Bar = 50 μm. |
|
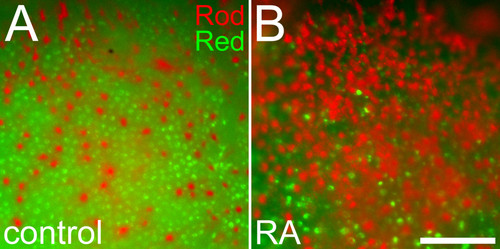
Altered positioning of rods in relation to red cones in response to retinoic acid treatment. Embryos treated with DMSO (A) or 0.3 μM RA (B) at 36 hpf and fixed at 60 hpf were doubly hybridized as whole mounts with probes corresponding to rod opsin (red color) and red cone opsin (green color). Following RA treatment, rods are more abundant, cones are more sparsely distributed, and rods display spacing characteristics more typical of cones. Bar = 50 μm. |
|
Expression of rod and cone transducin genes is altered by retinoic treatment beginning at the time of retinal neurogenesis. Embryos were treated with DMSO (Cont.; A, D), or 0.3 μM RA (B, C, E) at 36 hpf, and at 72 hpf were hybridized as 4 μm cryosections with probes corresponding to rod transducin (gnat1; A, B, C), or cone transducin (gnat2; D, E). Dorsal is up in all panels. In control embryos gnat1 is expressed in the ventral patch of rod photoreceptors (arrow in A) and in dispersed cells on the dorsal side of the retina (arrowheads in A). In some RA-treated embryos gnat1 expression is more intense in the ventral patch (arrow in B) and in all RA-treated embryos gnat1 expression is found in more cells in the dorsal and central retina (arrowheads in B and C). The section in panel C is positioned more peripherally in the retina compared to sections in other panels. Control embryos show an even distribution of gnat2 expression (D), which is reduced in intensity in RA-treated embryos (E). Bar = 50 μm. |
|
Retinoic acid treatment during retinal neurogenesis does not result in photoreceptors of mixed phenotype. Control embryos were treated with DMSO (A, C), or 0.3 μM RA (B, D to F) at 36 hpf, and at 60 hpf were processed as 5 μm cryosections for indirect immunofluorescence with rod- and cone-directed markers. Boxed regions in each panel appear at higher magnification in the insets. (A, B) Embryos were stained with a polyclonal anti-red opsin antibody (red color, stains red opsin), and monoclonal antibody 1D1 (green color; specific for rod opsin). Sections from control (A) or from RA-treated (B) embryos showed no evidence of colabeling. (C, D) Embryos were stained with zpr1 (red color, labeling red and green-sensitive cones) and a polyclonal anti-rod opsin antibody (green color, stains rods as well as green cones). In control embryos (C), there are singly- as well as doubly-labeled cells, indicating the likely presence of red cones (red color), green cones (yellow color; arrowheads), and rods (green color, arrows). (D) In embryos treated with RA, there is reduced staining of red cones and green cones and more intense staining for rods. (E, F). In an RA-treated embryo, cells positive for zpr1 (E) and the rod/green cone marker zpr3 (F) are found in ectopic locations (arrowheads). IPL, inner plexiform layer. Bar = 50 μm. |
|
Retinoic acid treatment during retinal neurogenesis results in a significant increase in retinal cell death. Control embryos were treated with DMSO (A, C), or 0.3 μM RA (B, D) at 36 hpf, and either were stained live at 75 hpf with the cell death marker Acridine Orange and prepared as whole mounts for viewing (A, B), or were sectioned at 5 μm and processed for cell death detection with the TUNEL kit (C, D); dorsal is up in all panels. Control embryos showed very few dead/dying cells (arrowheads in A), and these were found predominantly in the inner nuclear layer (C), while those treated with RA (D) showed widespread cell death in the inner nuclear layer (INL), ganglion cell layer (GCL), and circumferential germinal zone (CGZ, D). However, little cell death was detected in the outer nuclear layer (ONL) (D). Bar = 50 μm for all panels. |
|
Proliferating cells in the retina can activate a reporter gene in response to RA. Embryos carrying the RARE-YFP transgene were treated with 0.3 μM RA at 36 hpf, and at 48 hpf were processed as 3 μm cryosections for indirect immunofluorescence with an anti-GFP antibody (green color; A) and a marker for mitotic cells, anti-phosphohistone H3 (red color; B); panel C shows the merged image. Dorsal is up. Asterisk indicates the ganglion cell layer (GCL). There are mitotic cells in the retina that are both negative (arrows) and positive (arrowheads) for YFP expression. (D) Numbers of singly-labeled (PH3+, YFP-) and doubly-labeled (PH3+, YFP+) mitotic cells were counted as a function of position in the retina in a sample of sections from RA-treated embryos. Cells counted in the developing ganglion cell layer were lumped together with those of the inner nuclear layer (INL). Single-labeled anti-PH3 cells are found largely in the outer nuclear layer (ONL) and INL. Few single-labeled anti-PH3 are seen in the GZ. Colabeled cells are found evenly distributed among these retinal regions. Bar = 50 μm. |
|
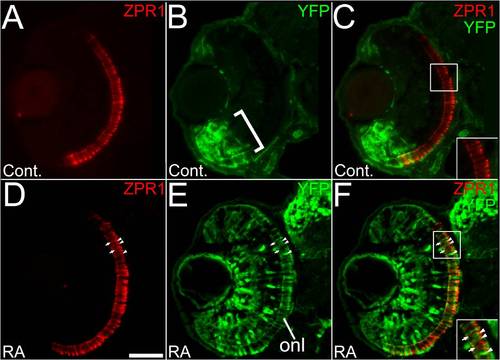
Retinoic acid signaling within a subpopulation of rod photoreceptors in response to prolonged retinoic acid treatment. (A to C) Embryos carrying the RARE-YFP transgene were treated with DMSO (A to C) or 0.3 μM RA (D to F) from 36 to 60 hpf, and were processed as 4 um cryosections for indirect immunofluorescence with an anti-GFP antibody (green) and antibody to rod opsin (1D1; red). (A) Rod photoreceptors are found densely populated in a ventral patch (arrow) and more widely distributed in the dorsal retina. (B) In control embryos, endogenous reporter expression is limited to cells of the ventral retina, but found in all retinal layers in that region (bracket). (C) Merged panel from A and B showing some colocalization of YFP and rod opsin (arrowheads) in the ventral retina, and none in the dorsal retina (inset). (D) Rod photoreceptors in a retina from an embryo treated with RA. Arrow indicates the ventral retina. (E) In experimental embryos the RA treatment leads to widespread expression of YFP, including the ONL. (F) Merged panel from D and E, showing rods in the dorsal retina colocalized with YFP (arrowheads) as well as those not expressing YFP (arrow). Bar = 50 μm. |
|
Multiple retinal cell types engage in retinoic acid signaling in response to retinoic acid treatment during retinal differentiation. (A to E) Embryos carrying the RARE-YFP transgene were treated with 0.3 μM RA at 48 hpf, and at 75 hpf were processed as 5 μm cryosections for indirect immunofluorescence with an anti-GFP antibody (green color in all panels) and the following markers (red color in all panels): zpr1 (stains red and green-sensitive cones; (A), zpr3 (stains rods and green cones; (B), zpr2 (stains RPE; C), zrf1 (stains Müller glia; D), and anti-PKC (stains rod bipolar cells; E). Dorsal is up in all panels; boxed regions in each panel appear at higher magnification in the insets. All types of retinal cells examined are capable of responding directly to RA by activating the RARE-YFP transgene (doubly-labeled cells in each panel; arrowheads; colabeling within the limit of resolution of our objective lens = 1.4 μm), although not all cells of each type respond (singly-labeled cells; arrows). (F) Embryos carrying the RARE-YFP transgene were treated with 0.3 μM RA at 72 hpf, and were fixed at 75 hpf as 5 μm cryosections for indirect immunofluorescence with the anti-GFP antibody. Widespread transgene expression indicates rapid response to RA. Bar = 50 μm. |
|
Specific retinoic acid receptors are expressed in the embryonic zebrafish retina. Wild-type, untreated embryos were fixed at 36 hpf (A, E), 48 hpf (B, F), 55 hpf (C, G), or 72 hpf (D, H), and were hybridized as 3 μm cryosections with probes corresponding to RXRγ (A-D) or RARαb (E-H); dorsal is up in all panels. (A to D) In younger embryos, RXRγ is expressed at the outer edge of ventral retina (arrow in A), and in scattered cells in inner and outer retina (arrowheads in A). In older embryos this expression becomes restricted to the emerging outer nuclear layer (ONL, B and C), and then to the most peripheral cells of the outer nuclear layer (black arrows in D) and periodically distributed cells of the inner nuclear layer (arrowheads in D). E.-H. In younger embryos, RARαb is expressed at the inner edge of ventral retina (arrows in E), and in older embryos shows widespread expression throughout the retina, with strongest hybridization signals in the ganglion cell layer (GCL) (F-H). The inset in F shows a representative control experiment using "sense" strand RARαb cRNA. Bar = 50 μm. |
|
Endogenous retinoic acid signaling and production of rods is reduced by knockdown of RARαb expression. (A, B) RARE-YFP embryos at 75 hpf. YFP expression was detected on embryos sectioned at 4 μm using an anti-GFP antibody. (A) In uninjected RARE-YFP embryos, there is robust expression of YFP in cells in the ventral retina (bracket). Non-specific antibody staining is indicated by asterisks. (B) RARE-YFP embryos injected with the rarαb/p53 MO show a marked reduction in the number of cells expressing YFP in the ventral retina (bracket). (C, D) Wildtype (SH) embryos at 75 hpf. (C) Tissue section from uninjected wildtype embryo labeled with a rod opsin antibody (1D1). Arrowhead: optic nerve head. (D) Injection of the rarαb/p53 MO into wildtype embryos results in reduced expression of 1D1 rod opsin antigen. (E) Numbers of rod photoreceptors were quantified by counting them on sections (see Methods). Wildtype embryos injected with the rarαb/p53 MO (n = 8, average 8 sections per eye) at 75 hpf had a significant reduction in the number of rods per section compared to uninjected embryos (n = 6, average 10 sections per eye); p < 0.5, Student′s T-Test). Bar = 50 μm. |
|
The effects of exogenous retinoic acid on RARαb morphants. (A, B) RARE-YPF embryos injected with rarαb/p53 MO and fixed at 60 hpf. YFP expression was detected on 4 μm cryosections using an anti-GFP antibody. (A) RARE-YFP embryos injected with the rarαb/p53 MO show reduced expression of YFP in the ventral retina. (B) RARE-YFP embryo injected with rarαb/p53 MO and treated with RA beginning at 36 hpf. Upregulation of YFP expression is evident in cells across all the retinal layers. (C, D) Wildtype (SH) embryos injected with rarαb/p53 MO and fixed at 60 hpf, sectioned at 4 μm, and labeled with an antibody to rod opsin (1D1). (C) Embryo injected only with rarαb/p53 MO. (D) Embryo injected with rarαb/p53 MO and treated with RA beginning at 36 hpf. (E) Numbers of rod photoreceptors were quantified by counting them on sections (see Methods). Statistically significant differences between DMSO and RA-treated groups were not detected in wildtype RARαb morphants. (DMSO group n = 8, average 9 sections per eye; RA group n = 9, average 8 sections per eye) (n = 13, average 8 sections per eye). Bar = 50 μm. (F) Numbers of rod photoreceptors per section in embryos treated with the p53 MO only, and exposed to DMSO (n = 4, average 9 sections per eye) or RA (n = 5, average 8 sections per eye), and examined at 63 hpf. In this experiment RA treatment results in significantly higher numbers of rods. Bar = 50 μm. |
|
Effects of a short term exposure to retinoic acid on retinoic acid signaling and rod photoreceptors. Embryos carrying the RARE-YFP transgene were treated with either DMSO (A and C) or 0.3 μM RA (B and D) from 36 to 39 hpf. (A and B) Embryos were processed at 49 hpf as 4 μm cryosections for anti-GFP indirect immunofluoresence. (A) Control embryos exhibiting endogenous transgene expression in the ventral retina. (B) Embryos treated with the ′pulse′ of RA show strong ventral labeling, as well as very weak labeling elsewhere in the retina. (C and D) Embryos were processed at 60 hpf for whole mount in situ hybridization with a probe directed against rod opsin mRNA. (C) Control embryos show the normal distribution of rods across the retina. (D) Embryos treated with the RA ′pulse′ show a similar distribution and density of rod photoreceptors. Bar = 50 μm. |
|
Retinoic acid signaling within a subpopulation of red- or green-sensitive cone photoreceptors in response to prolonged retinoic acid treatment. (A to C) Embryos carrying the RARE-YFP transgene were treated with DMSO (A to C) or 0.3 μM RA from 36 to 60 hpf, and processed as 4 μm cryosections for indirect immunofluorescence with an anti-GFP antibody (green) and the antibody zpr1 which labels both red- and green-sensitive cones. (A) In control embryos red and green-sensitive cones are found widely distributed in the retina. (B) In control embryos, endogenous reporter expression is limited to cells of the ventral retina, (bracket) but found in all retinal layers in that region. (C) Merged panel from A and B showing no colocalization with YFP in the dorsal retina (inset). (D) A retina from an embryo treated with RA. (E) In experimental embryos, the RA treatment leads to widespread expression of YFP, including many cells in the ONL. (F) Merged panel from D and E, showing some cones in the dorsal retina expressing YFP (inset, arrowheads) and many that do not express YFP (inset, arrows). Bar = 50 μm. |