- Title
-
Post-embryonic nerve-associated precursors to adult pigment cells: genetic requirements and dynamics of morphogenesis and differentiation
- Authors
- Budi, E.H., Patterson, L.B., and Parichy, D.M.
- Source
- Full text @ PLoS Genet.
|
Post-embryonic expression of embryonic neural crest and glial markers. Shown are in situ hybridizations performed on transverse sections of 7–9 SSL larvae. (A) foxd3 transcript was detected in dorsal root ganglia (arrow) and scattered cells (e.g., arrowheads) within the myotome (m) and near the epidermis (e). (B) sox10 expression by cells adjacent to the neural tube (arrow) and within the myotome (arrowhead). (C) foxd3+ cells (e.g., arrowheads) at the base of the dorsal fin (f). (D) foxd3+ cells within the myotomes and near the epidermis. (E–G) foxd3+, sox10+, and crestin+ cells within the myotomes. (H–J) Cells expressed mitfa (arrowheads), within the horizontal myoseptum (H), at the surface of the myotome (I), and at the base of anal fin (J) (see text for details). |
|
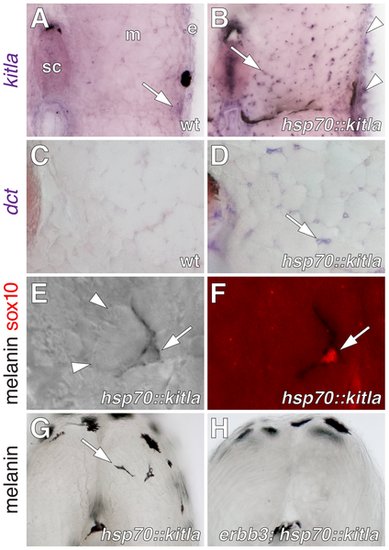
kitla misexpression induced ectopic melanophores within the myotomes. (A) kitla was normally expressed within the epidermis and hypodermis (arrow) during post-embryonic development of wild-type fish. sc, spinal cord. m, myotome, e, epidermis. (B) In sibling Tg(hsp70::kitla) larvae, heat shock resulted in increased kitla transgene expression within the epidermis (arrowheads) as well as ectopic expression within the myotomes (arrow), spinal cord, and elsewhere. (C) The late melanophore lineage marker dopachrome tautomerase (dct), encoding an enzyme required for melanin synthesis [96], [97], was not expressed within the myotomes of wild-type fish. (D) However, dct was expressed by scattered cells within the myotomes (arrow) in larvae misexpressing kitla. (E,F) Newly differentiated ectopic melanophores (arrow) were found between myotubes (arrowheads; E) and these cells continued to express sox10 protein (F). (G) Vibratome section revealing ectopic melanophores (arrow) within the myotome of a Tg(hsp70::kitla) larva 48 h after the initiation of kitla misexpression. Melanophores deep within the myotome were found only in Tg(hsp70::kitla), though melanophores were occasionally found within the horizontal myoseptum of both transgenic and wild-type larvae [ectopic melanophores per larva, Tg(hsp70::kitla): mean±SE = 1.3±0.15, range = 0–7 cells, n = 80 larvae; non-transgenic siblings: mean±SE = 0±0, range = 0, n = 69]. Longer durations of kitla misexpression resulted in more ectopic melanophores per larva. Suggesting that ectopic melanophores differentiated in situ rather than migrated into the myotomes from the hypodermis, labeling of hypodermal cells by photoconversion of mitfa::Eos+ [55] failed to reveal movement of cells away from enhanced kitla expression in the epidermis into the myotome (n = 10 larvae, 3–5 cells per individual). (H) In contrast to the wild-type, ectopic melanophores were significantly fewer in erbb3b; Tg(hsp70::kitla) mutants [Wilcoxon test, Z = 7.1, P<0.0001; ectopic melanophores per larva, erbb3b; Tg(hsp70::kitla): mean±SE = 0.04±0.03, range = 0–1, n = 50 larvae; non-transgenic siblings: mean±SE = 0±0, range = 0, n = 70] and in Tg(hsp70::kitla) larvae treated with AG1478 during the ErbB embryonic critical period [Wilcoxon test, Z = 2.9, P<0.005; ectopic melanophores per larva, AG1478-treated Tg(hsp70::kitla): mean±SE = 0.7±0.2, range = 0–4, n = 45 larvae; untreated Tg(hsp70::kitla) siblings: mean±SE = 1.6±0.2, range = 0–5, n = 35]. |
|
Extra-hypodermal cells expressing mitfa::GFP, foxd3, and sox10 in wild-type larvae and their deficiency in erbb3b mutants. All images are from early metamorphic (6.2–8.0 SSL) wild-type larvae except for B, L, and M, from erbb3b mutant larvae. (A–C) Transverse confocal projections (collapsing ~1.5 mm of trunk along the anterior-posterior axis) showing GFP+ cells in living larvae (left side of each larva is shown). Images correspond to Videos S1, S2, S3. (A) mitfa::GFP+ cells in a wild-type fish occur in the hypodermis, between the epidermis (e) and the myotome (m), within the the myotome itself (arrow), and above the spinal cord (sc). Arrowhead, location of the horizontal myoseptum. (B) In erbb3b mutants, most mitfa::GFP+ cells were missing. This image is intentionally overexposed compared to A, revealing faint reflected fluorescence from iridescent iridophores in the hypodermis (arrowhead), which are present in wild-type larvae as well. (C) sox10::GFP+ cells were found in extra-hypodermal locations of wild-type Tg(-4.9sox10:egfp)ba2 larvae [98]. (D–M) Immunohistochemical analyses of fixed specimens. (D) Co-expression of mitfa::GFP (green) and sox10 (red). v, vertebral column. (E) mitfa::GFP+ cells aligned with mbp+ glia (red) along ventral root motor fibers. Arrow, mitfa::GFP+ cells did not co-express mbp. (F) Lateral view showing mitfa::GFP+ cells and foxd3+ cells (red) between Hu+ neurons (blue) of dorsal root ganglia. mitfa::GFP+ and foxd3+ cells were often found close to one another (e.g., arrows) whereas other cells co-expressed mitfa::GFP and foxd3 (arrowhead). (G) Lateral view with superimposed brightfield and fluorescence images showing mitfa::GFP+ and foxd3+ cells along a peripheral nerve fiber stained for acetylated alpha tubulin (blue) within the myotome. Arrows, adjacent mitfa::GFP+ and foxd3+ cells. (H). A nerve plexus near the base of the caudal fin harbored numerous mitfa::GFP+ and foxd3+ cells. (I) Along a peripheral nerve within the myotome some cells co-expressed mitfa::GFP and foxd3 (arrowhead), whereas cells expressing either mitfa::GFP+ or foxd3+ were often juxtaposed (arrows). (J,K) Transverse sections through the dorsal trunk showing sox10+ cells (J) and foxd3+ cells (K) n the hypodermis, within the myotomes, and near the spinal cord. Arrow, lateral line nerve. (L,M) In erbb3b mutant larvae, very few sox10+ (L) or foxd3+ (M) cells were found. |
|
Proliferative extra-hypodermal cells revealed by post-embryonic EdU incorporation. All images from transverse sections of wild-type larvae staged as in Figure 3 (see Figure S4 for comparisons with erbb3b mutant larvae). (A,B) Merged images showing cells (arrowheads) within the myotomes labeled for either mitfa::GFP (red in A) or sox10::GFP (red in B) as well as EdU (green). (C,D) Cells within the lateral myotomes (m) and near the hypodermis (C) or at the base of the anal fin (f in D) labeled for mitfa::GFP (red), foxd3 (blue), and EdU (green). Merged views show fluorescence images or fluorescence images with brightfield overlays. Arrowheads, triple-labeled cells. m, myotome. e, epidermis. |
|
DiI-labeling showed extra-hypodermal contributions to metamorphic melanophores and iridophores. (A–C) DiI labeled tissues imaged immediately after injection into the base of the dorsal fin (A), the vicinity of the horizontal myoseptum and lateral line nerve (B), and the inner myotome (C). Each site yielded hypodermal DiI+; mitfa::GFP+ cells or DiI+ melanophores (12 of 30 larvae, 3 of 30 larvae, 15 of 87 larvae, respectively). (D–F) DiI+ cells that expressed either mitfa::GFP (D) or contained melanin (E,F) found within the lateral hypodermis 4 d following injection into the base of the dorsal fin (D, F) or the inner myotome (E). (G) DiI-labeling was observed for additional cells including iridophores. Although the frequencies with which DiI labeled pigment cells were found differed between injection sites, each site gave rise to DiI+ iridophores at a frequency indistinguishable from that of DiI+ mitfa::GFP+ cells (χ2 = 0.6, d.f. = 1, P = 0.4). We did not observe DiI-labeled xanthophores. |
|
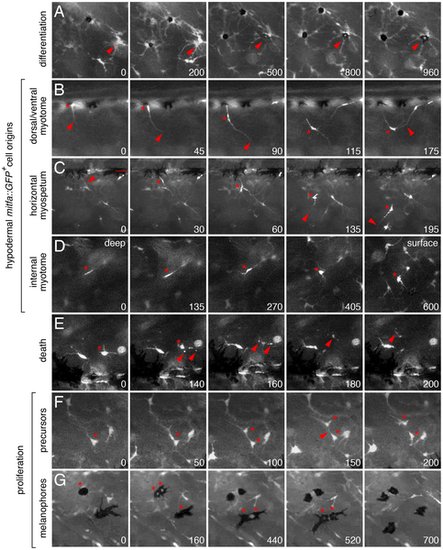
Ex vivo time-lapse imaging revealed extra-hypodermal origins and morphogenetic behaviors of hypodermal mitfa::GFP+ cells and melanophores. All panels show lateral views of larval trunks and are derived from time-lapse movies of Tg(mitfa::GFP) larvae. Elapsed time (min) at lower right of each panel. (A) mitfa::GFP+ cells differentiated into melanophores (e.g., arrowhead). (B–D) mitfa::GFP+ cells entered the hypodermis during the larval-to-adult transformation. (B) A cell at the dorsal margin of the myotomes extended a long process (arrowhead) into the hypodermis and interacted with processes of a second cell. *, cell body. (C) A long process (arrowhead) preceded emergence of the cell body (*) from the level of the horizontal myoseptum (dotted line). This cell subsequently interacted with a neighboring cell, extended a processes ventrally, and moved in that direction. (D) A cell initially deep within the myotome (*) emerged into the hypodermis. The focal plane changes across panels, from deep within the myotome to the surface of the myotome and hypodermis, where other cells are found already. (E) Death of mitfa::GFP+ cell (*) revealed by fragmentation and cellular debris (arrowheads). (F,G) mitfa::GFP+ cells (F) and melanophores that retain some residual GFP expression (G) proliferating within the hypodermis. Melanophores in G are imaged in a kita mutant (see text for details). See Videos S4, S5, S6, S7, S8, S9, S10, S11, S12, S13, S14, S15, S16. |
|
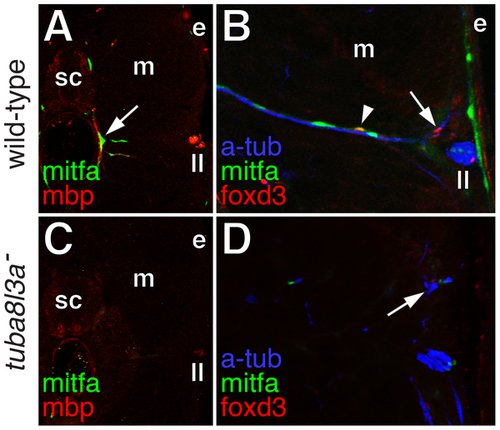
Extra-hypodermal precursors were deficient in tuba8l3a mutant larvae. (A) Wild-type larvae exhibited mitfa::GFP+ cells (green) associated with mbp+ glia (red) of peripheral nerves (arrow). sc, spinal cord; m, myotome; e, epidermis; ll, lateral line nerve. (B) mitfa::GFP+ cells (green), foxd3+ cells (red; arrow), and doubly labeled mitfa::GFP+; foxd3+ cells (arrowhead) were associated with nerve fibers stained for acetylated alpha tubulin (blue). (C) In tuba8l3a mutants, regions deficient for mbp+ glia were also deficient for mitfa::GFP+ cells. (D) Peripheral nerves were often defasciculated (arrow) and were deficient for foxd3+ and mitfa::GFP+ cells. |
|
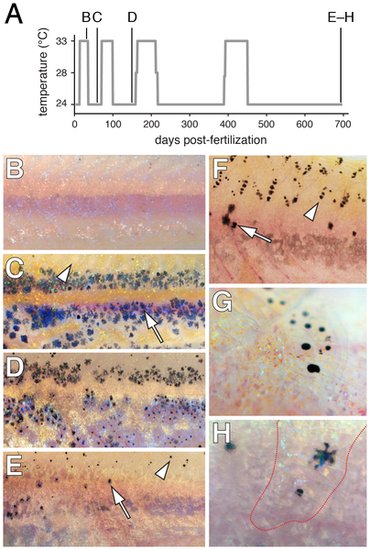
Limited regeneration of adult hypodermal melanophores following genetic ablation. (A) Time-course of temperature shifts, with letters corresponding to images in B–H. Final sample size at 698 days post-fertilization: n = 5. (B) A young adult kita; csf1rTS mutant at 33°C lacked melanophores as in kita; csf1rj4e1 mutants [38] (some melanized cellular debris resulting from melanophore death is evident dorsally). (C) Temperature downshift to 24°C allowed recovery of a hypodermal melanophore (e.g., arrow) complement initially indistinguishable from kita single mutants [59]. Arrowhead, the dorsal flank is initially devoid of scale-associated melanophores, as is typical of kita mutants. (D,E) Additional rounds of ablation and recovery yield progressively fewer hypodermal melanophores (arrow), though some melanophores develop on the dorsal scales (arrowhead). Hypodermal xanthophores and iridophores were depleted as well (data not shown). (F) Dorsal flank of another individual showing hypodermal melanophores (arrow) and scale melanophores (arrowhead). (G) Detail of scale-associated melanophores. Iridescent iridophores and yellow-orange xanthophores are apparent as well. (H) Detail of hypodermal melanophores viewed through an overlying scale containing a concentration of xanthophores and iridophores (outlined in red). |
|
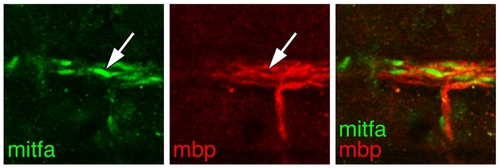
mitfa::GFP+ cells amongst glia of the lateral line nerve. Sagittal view of a wild-type larva showing mitfa::GFP+ cells (mitfa, green; arrow) aligned with mbp+ glia (red) of the main trunk lateral line nerve, near the horizontal myoseptum. |
|
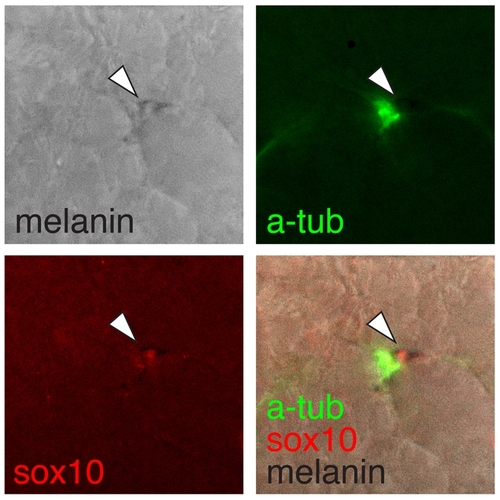
Ectopic kitla-responsive melanogenic cells were nerve-associated. Shown is an ectopic sox10+ (red) melanophore (arrow) within the myotome adjacent to a nerve fiber stained for acetylated tubulin (green). |
|
Defects in wild-type larvae treated with ErbB inhibitor AG1478 during the erbb3b embryonic critical period. (A,B) Post-embryonic mbp+ glia (red; arrowheads) were reduced though not eliminated in AG1478-treated larvae. Inset, mitfa::GFP+ cell (green) aligned on mbp+ glia of a peripheral nerve at the level of the horizontal myoseptum. sc, spinal cord; m, myotome; e, epidermis; ll, lateral line nerve. (C,D) foxd3+ cells (red; arrowheads) within the myotome were missing from AG1478-treated larvae. |
|
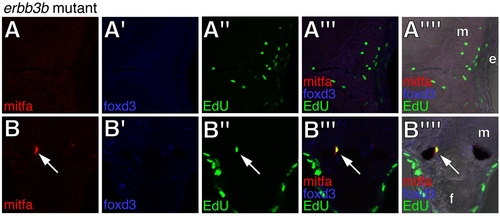
Deficiencies in mitfa::GFP+ cells, foxd3+ cells, and EdU incorporation in erbb3b mutants. (A,B) Views and annotations correspond to those for wild-type larvae in main text Figure 5. Arrow, a rare EdU+; mitfa::GFP+ cell at the base of the ventral fin. |
|
erbb3b-dependence of kita-independent hypodermal melanophores. (A) kitab5 presumptive null allele [59] with stripes of kita-independent hypodermal melanophores. (B) Fish doubly mutant for kitab5 and the presumptive null allele erbb3but.r2e1 showing loss of many kita-independent melanophores as well as gaps in the “interstripes” (e.g., arrowhead) reflecting an iridophore deficiency. Patches of residual melanophores may be of clonal origin. |
|
mitfa::GFP+ cells in adult fish. Shown are cross-sections through ~20 SSL (~80 days post-fertilization) adult wild-type fish. (A) A persisting nerve-associated mitfa::GFP+ cell (green; arrow). sc, spinal cord; m, myotome. (B) mitfa::GFP+ cells (green; arrowhead) within the lateral line nerve. e, epidermis (C) mitfa::GFP+ cells (green; arrow) and foxd3+ cells (red; arrowhead) in the ventral myotomes and base of the anal fin (f). (D) mitfa::GFP+ cell (green; arrow) and doubly labeled mitfa::GFP+; foxd3+ cell (arrowhead) associated with an adult scale (s). |