- Title
-
Retinoic acid receptor signaling regulates choroid fissure closure through independent mechanisms in the ventral optic cup and periocular mesenchyme
- Authors
- Lupo, G., Gestri, G., O'Brien, M., Denton, R.M., Chandraratna, R.A., Ley, S.V., Harris, W.A., and Wilson, S.W.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
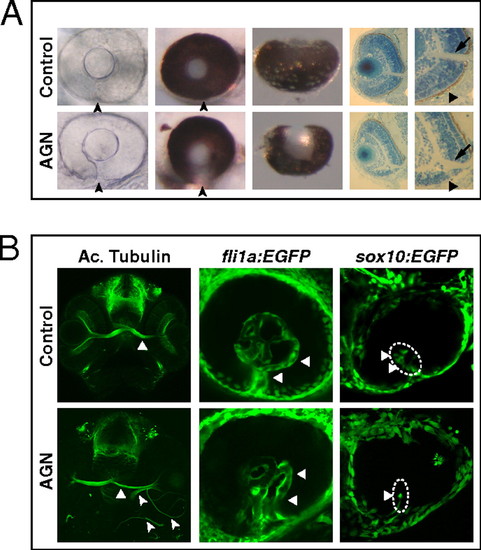
Inhibition of RAR signaling causes coloboma in zebrafish. (A) Coloboma (arrowheads) in embryos treated with 2.5–5 μM AGN from 3 to 6 s. (Left) Lateral view of 48-hpf phenylthiourea-treated embryos. (Center) Lateral (Center Left) and ventral (Center Right) views of 60-hpf eyes. (Right) Sections of 60-hpf eyes showing the lack of retinal pigmented epithelium (triangles) and a shortening of the retina ventral to the optic nerve head (arrows) in the AGN-treated embryo. (B, Left) Immunostaining with an anti-acetylated (Ac.) tubulin antibody at 48–50 hpf, showing abnormal retinal axon trajectories in the AGN-treated embryo (arrowheads), besides the normal contralateral projection (triangles). (Center) fli1a:EGFP transgenic embryos at 48 hpf. The AGN-treated embryo exhibits disrupted vascularization (triangles). (Right) sox10:EGFP transgenic embryos at 32–36 hpf. The AGN-treated embryo shows less POM cells within the choroid fissure (triangles). |
|
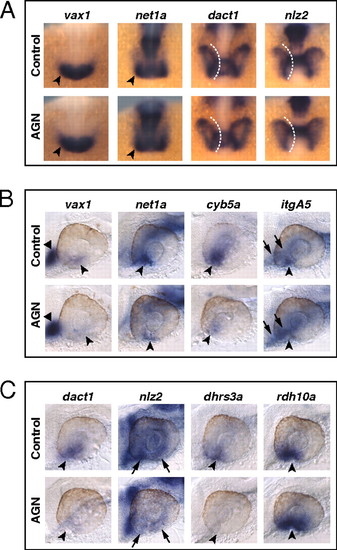
RAR signaling regulates gene expression in the VR/OS. (A–C) Embryos treated with DMSO or 10 μM AGN from 3s and hybridized at 18s (A) or 24–25 hpf (B and C) with the indicated probes. (A) Dorsal/anterior views of heads. AGN treatment has no major effects on the expression of vax1 and net1a at the optic vesicle/forebrain junction (arrowheads) and of dact1 and nlz2 in the optic vesicle. Dashed lines highlight the proximal edge of the optic vesicle. (B and C) Lateral views of eyes. AGN treatment causes down-regulation of vax1, net1a, cyb5a, itgA5, dact1, nlz2, and dhrs3a and up-regulation of rdh10a in the VR/OS (arrowheads). Triangles point to vax1 expression in the ventral forebrain (B). Arrows point to itgA5 expression in extraocular tissue at the back of the eye (B) and nlz2 expression on each side of the choroid fissure (C). Panels are representative images of the following numbers (n) of independent experiments: vax1, net1a, n = 4; itgA5, n = 3; and cyb5a, dact1, nlz2, dhrs3a, rdh10a, n = 2. Approximately 15–30 embryos were assayed for each condition and probe in each experiment. EXPRESSION / LABELING:
|
|
RAR signaling regulates gene expression in POM. (A–C) Embryos treated with DMSO or 10 μM AGN from 3s and hybridized at 18s (A), 24–25 hpf (B), or 31 hpf (C) with the indicated probes. (A) Dorsal/anterior views. In the AGN-treated embryos, foxC1a and nlz1, but not twist1a, are down-regulated in migrating POM (arrowheads). (B and C) Lateral views of eyes. AGN treatment decreases foxC1a, nlz1, eya2, pitx2, and lmx1b1 expression in both anterior-ventral (arrowheads) and anterior-dorsal (black triangles) POM. AGN treatment decreases twist1a expression in dorsal POM (black triangles). Expression of pitx2 and lmx1b1 within the choroid fissure is also diminished (white dashed circles and triangles). Residual foxC1a, eya2, and lmx1b1 staining is detectable in a ventro-medial location behind the eye (arrows). pitx2 is expressed in extraocular mesodermal tissue in both the control and the AGN-treated embryo (arrows). Panels are representative images of the following numbers of independent experiments: pitx2, n = 4; foxC1a, n = 3; and nlz1, twist1a, eya2, lmx1b1, n = 2. Approximately 10–30 embryos were assayed for each condition and probe in each experiment. EXPRESSION / LABELING:
|
|
Quantification of gene expression changes after manipulations of RAR signaling. (A and B) Real-time PCR quantification of gene expression in 31- to 33-hpf dissected heads (A) or 25- to 28-hpf dissected heads and whole embryos (B) after treatments with the indicated reagents from 3s, shown as the mean ratio between AGN or RA and DMSO conditions in six (A) or three (B) biological replicates. Error bars show SDs. *P < 0.05; **P < 0.01; ***P < 0.001; ns, nonsignificant (P ≥ 0.05) according to two-tailed Student's t test. |
|
Exogenous RA activates genes expressed in the VR/OS and in POM. (A and B) Lateral views of eyes from 31-hpf hybridized embryos treated with DMSO or 1 μM RA from 18s. (A) Arrowheads point at the expression of vax1, dact1, nlz2, and itgA5 in the VR/OS. RA treatment reduces vax1 expression in the VR/OS while inducing it ectopically in the dorsal telencephalon (triangle) and diencephalon (arrow). The RA-treated embryos show dact1 and nlz2 expression throughout the retina and ectopic itgA5 expression in the dorsal retina (triangle). (B) RA treatment increases foxC1a, nlz1, lmx1b1, and pitx2 expression in anterior-ventral (arrowheads) and/or anterior-dorsal (triangles) POM. Approximately 20 embryos for each condition and probe were used for these analyses. EXPRESSION / LABELING:
|
|
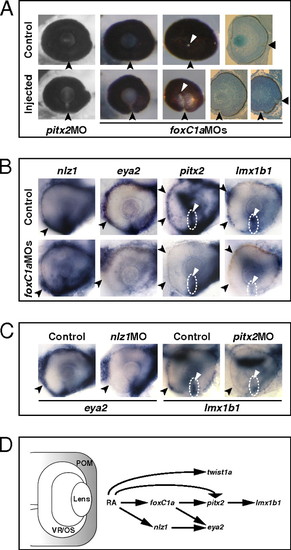
Abrogation of FoxC1a, Nlz1, and Pitx2 function causes coloboma and affects gene expression in POM. (A) Eyes of embryos injected with pitx2 MO or foxC1a MOs showing coloboma at 72 hpf, as detected in lateral view (Left and Center Left), posterior view (Center Right), and histological sections (Right). The injected eyes exhibit an open choroid fissure (arrowheads) ventral to the optic disk (triangles). (B and C) Lateral views of 32-hpf eyes. Panels are representative images of two to three independent experiments. Approximately 10–20 embryos were used for each condition and probe in each experiment. (B) Decreased expression of eya2, pitx2, and lmx1b1, but not nlz1, in choroid fissure POM (dashed circles and triangles) and/or anterior POM (arrowheads) in the foxC1a MO-injected embryos. (C) Decreased expression of eya2 in anterior POM (arrowheads) of an nlz1 MO-injected embryo and of lmx1b1 in anterior POM (arrowheads) and within the choroid fissure (dashed circles and triangles) of a pitx2 MO-injected embryo. (D) Proposed model of the molecular mechanisms of RAR-dependent RA signaling during development of the zebrafish POM (highlighted in gray). See text for details. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Pax2 regulates some VR/OS genes in parallel to RAR signaling. (A and B) Lateral views of 25- to 26-hpf eyes. Approximately 15–20 control or mutant embryos for each probe were used for these analyses. (A) The noi embryos show down-regulation of net1a, cyb5a, and itgA5, but not vax1, in the VR/OS (arrowheads). Arrows point to itgA5 expression in extraocular tissue at the back of the eye. (B) Expression of dact1, nlz2, dhrs3a, and rdh10a in ventral eye cells is not affected in the noi mutants (arrowheads). Triangles point to nlz2 dorsal retinal expression. (C) Proposed model of the molecular mechanisms of RAR signaling in the developing zebrafish VR/OS (highlighted in gray). See text for details. EXPRESSION / LABELING:
|
|
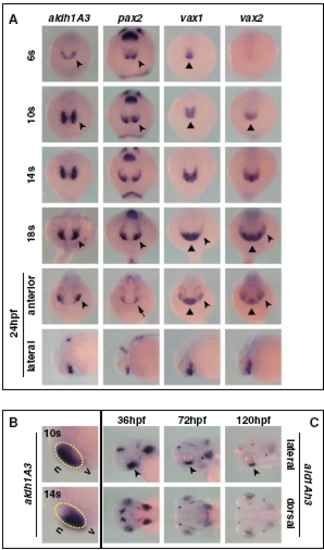
Expression pattern of aldh1A3 during zebrafish development. (A) Frontal/dorsal (6s–18s and 24 hpf, top row) or lateral (24 hpf, bottom row) views of wild-type embryos hybridized with the indicated probes, showing that aldh1A3 is expressed at the right time and place to take part in ventral optic cup morphogenesis by comparison with other known regulators of ventral eye development. During early somitogenesis, expression of aldh1A3 and pax2 becomes detectable in the eye field (arrowheads), whereas vax1 and vax2 expression is limited to the forebrain (triangles). By 14s–18s, all these genes are expressed in the ventral eye (arrowheads), while vax1 and vax2 remain also expressed in the ventral forebrain (triangles). At 24 hpf, aldh1A3, vax1, and vax2 are expressed in the ventral optic cup (arrowheads), while pax2 expression is confined to the optic stalk (OS) (arrow). Similar to previous stages, vax1 and vax2 domains also include the ventral forebrain (triangles). (B) Lateral views of the head region of 10s or 14s embryos, showing that aldh1A3 expression is initially detectable in the nasal (n) part of the eye but becomes localized to the ventral (v) eye as early as 14s. The yellow dashed circles highlight the eye region. (C) Lateral (top row) or dorsal (bottom row) of 36- to 120-hpf embryos, showing that aldh1A3 expression is maintained in the ventral retina (VR)/OS throughout the stages of optic fissure closure (arrowheads). |
|
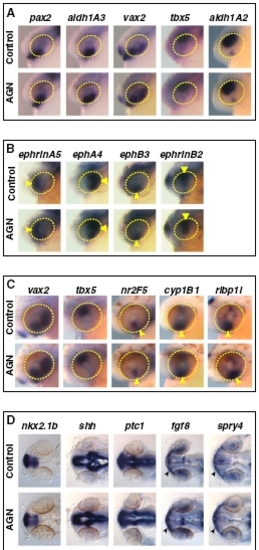
RAR signaling is not required for patterning of the retina and forebrain. Embryos treated with DMSO or 10 μM AGN from 3s and hybridized at 24–25 hpf (A, B, and D) or 31 hpf (C) with the indicated probes. (A–C) Lateral views of heads. Yellow circles highlight the eye region. (B) Arrowheads and triangles point to the expression of ephrinA5 and ephA4 in the nasal or temporal retina, respectively, or to the expression of ephB3 and ephrinB2 in the dorsal retina and VR, respectively. (C) Arrowheads point to the expression of nr2F5, cyp1B1, or rlbp1l in the VR/OS. (D) Dorsal views of heads. Arrowheads point to fgf8 or spry4 expression in the OS. |
|
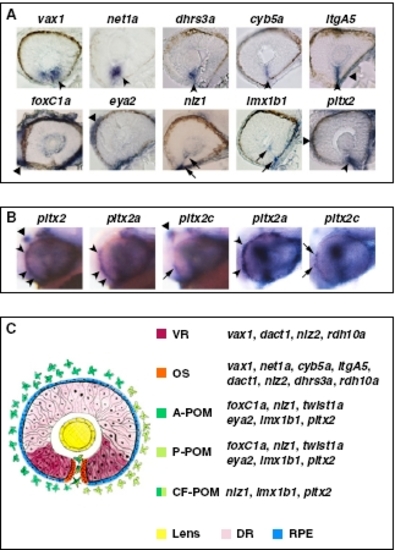
Expression patterns of VR/OS and POM genes. (A) Histological sections of 32- to 36-hpf eyes hybridized with the indicated probes. In the upper row, arrowheads point to expression in VR/OS cells, and the triangle points to itgA5 in extraocular tissue. In the lower row, triangles point to expression in POM around the optic cup, arrows point to expression in POM cells inside the choroid fissure, and the arrowhead points to pitx2 expression at the level of the choroid fissure, which might include OS cells besides POM. (B) Lateral views of 31-hpf heads (left three images) or eyes (right two images) hybridized with a pitx2 pan-isoform probe or with pitx2a- or pitx2c-specific probes, showing that pitx2a is the predominant isoform in the POM (arrowheads), whereas pitx2c is selectively expressed in the dorsal diencephalon (triangles). Arrows point to limited expression of pitx2c in few POM cells. (C) Schematic representation of the expression domains of VR/OS and POM genes, based on the data shown in A, Figs. 2 B and C and 3 B and C, and Fig. S5 A and B. A-POM, anterior POM; P-POM, posterior POM; CF-POM, POM located within the choroid fissure; DR, dorsal retina; RPE, retinal pigmented epithelium. |
|
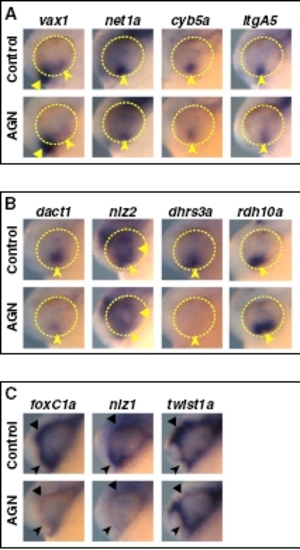
RAR signaling regulates gene expression in the VR/OS and in POM. Lateral views of heads of 24- to 25-hpf embryos treated with DMSO or 10 μM AGN from 3s and hybridized with the indicated probes. (A and B) AGN treatment causes down-regulation of vax1, net1a, cyb5a, itgA5, dact1, nlz2, and dhrs3a and up-regulation of rdh10a in the VR/OS (arrowheads). Triangles point to expression of vax1 in the ventral forebrain (A) and nlz2 in the dorsal retina (B). (C) AGN treatment decreases foxC1a and nlz1 expression in both anterior-ventral (arrowheads) and anterior-dorsal (triangles) POM as well as twist1a expression in dorsal POM only (triangles). |
|
RAR signaling regulates VR/OS and POM genes and is required beyond 18s to maintain their expression. Lateral views of eyes of 31-hpf hybridized embryos treated with DMSO or 10 μM AGN from 3s (A and B) or from 18s or 23 hpf (23h) (C and D), as indicated. (A) AGN treatment causes down-regulation of vax1, net1a, cyb5a, and itgA5 in VR/OS cells (arrowheads). Triangles point to vax1 expression in the ventral forebrain. Arrows point to itgA5 expression in extraocular tissue at the back of the eye. (B) In the control eye, dhrs3a is expressed in VR/OS cells (arrowhead), in the dorsal retina (triangle), and in dorsal POM (arrow). All these domains are down-regulated in the AGN-treated embryo. AGN treatment decreases foxC1a and nlz1 expression in both anterior-ventral (arrowheads) and anterior-dorsal (triangles) POM, although residual foxC1a staining is detectable in a ventro-medial location at the back of the eye (arrows). AGN treatment decreases twist1a expression in dorsal POM only (triangles). (C) Embryos treated with DMSO or AGN from 23 hpf show that AGN treatment causes down-regulation of vax1 and dhrs3a in the VR/OS (arrowheads) and of dhrs3a in the dorsal retina (triangle) and in dorsal POM (arrow). foxC1a and nlz1 expression in both anterior-ventral (arrowheads) and anterior-dorsal (triangles) POM is decreased in the AGN-treated embryos. (D) Embryos treated with DMSO or AGN from 18s or from 23 hpf showing decreased expression of both net1a and cyb5a in the VR/OS (arrowheads) only in the embryos treated with AGN from 18s. |
|
RAR signaling regulates gene expression in the VR/OS and in POM. Lateral views of heads of 31-hpf embryos treated with DMSO or 10 μM AGN from 3s and hybridized with the indicated probes. (A) AGN treatment causes down-regulation of vax1, net1a, cyb5a, and itgA5 in VR/OS cells (arrowheads). Triangles point to vax1 expression in the ventral forebrain. (B) In the control eye, dhrs3a is expressed in VR/OS cells (arrowhead), in the dorsal retina (triangle), and in dorsal POM (arrow). All these domains are down-regulated in the AGN-treated embryo. AGN treatment decreases foxC1a and nlz1 expression in both anteriorventral (arrowheads) and anterior-dorsal (triangles) POM as well as twist1a expression in dorsal POM only (triangles). (C) AGN treatment decreases eya2, pitx2, and lmx1b1 expression in anterior-ventral (arrowheads) and/or anterior-dorsal (black triangles) POM. Expression of lmx1b1 within the choroid fissure is also diminished (white triangles). pitx2 is strongly expressed in extraocular mesodermal tissue in both the controls and the AGN-treated embryo (arrows). |
|
Effects of exogenous RA on eye dorsoventral (DV) polarity. (A and B) Lateral views of heads of 25 to 26 hpf embryos treated with DMSO or 0.25 μM RA from 3s and hybridized with the indicated probes. RA treatment up-regulates pax2, but not vax1, net1a, vax2, or aldh1A3 expression in the dorsal eye. Arrowheads point to expression in the VR/OS. RA treatment causes no evident effects on tbx5 expression in the dorsal eye (triangles). |
|
Exogenous RA increases expression of VR/OS and POM genes. (A and B) Lateral views of eyes from 25- to 29-hpf hybridized embryos treated with DMSO or 0.125–0.5 μM RA from 3s. (A) In the control eyes, dhrs3a, nlz2, dact1, and itgA5 are expressed in VR/OS cells (arrowheads). nlz2 also has a restricted expression domain in the dorsal retina (triangle). RA treatments cause up-regulation of all these genes in the dorsal retina. Arrows point to partial dact1 and itgA5 up-regulation in the central retina region at lower RA doses. Dashed circles highlight the eye region in treated embryos. (B) RA treatments increase foxC1a, nlz1, lmx1b1, and pitx2 expression in anterior POM (triangles). The RA-treated embryos also show dose-dependent decrease in nlz1 and lmx1b1 expression in ventral POM (arrowheads). Arrows point to up-regulation of nlz1 in the dorsal eye region of the RA-treated embryos. |
|
Early response genes to up-regulated RA signaling. (A) Real-time PCR quantification of gene expression in 25- to 26-hpf whole embryos after embryo treatments with the indicated reagents from 3s, shown as the mean ratio between RA and DMSO conditions in 10 biological replicates. Error bars show SDs. ***P < 0.001; ns, nonsignificant (P e 0.05) according to two-tailed Student’s t test. (B and C) Embryos at 18s were incubated in DMSO or 0.5 μM RA for 30– 50 min and harvested for gene expression analysis by real-time PCR (B) or ISH (C). (B) Results of real-time PCR quantification shown as the ratio between RA and DMSO conditions and representing the mean of eight biological replicates for vax1, dact1, and foxC1a or six biological replicates for nlz2 and nlz1. Error bars show SDs. **P < 0.01; ***P < 0.001 according to two-tailed Student’s t test. (C) Dorsal/anterior views of heads of 18s whole embryos hybridized with the indicated probes. RA treatment enhances expression of nlz2 and dhrs3a in the optic vesicle and of foxC1a and nlz1 in migrating POM cells (arrowheads). White dashed lines highlight the proximal edge of the optic vesicle. |
|
Abrogation of FoxC1a, Nlz1, or Pitx2 function does not affect VR/OS gene expression. Lateral views of eyes from 31- to 32-hpf hybridized embryos showing no obvious changes in the expression of vax1, net1a, cyb5a, and itgA5 in the VR/OS (arrowheads) between the controls and the embryos injected with foxC1a MO (A), nlz1 MO (B), or pitx2 MO (C). |
|
Expression patterns of POM genes in POM gene morphants. (A) Frontal/dorsal views of 20s hybridized embryos showing no differences in the expression of nlz1 and twist1a in migrating POM (arrowheads) between the controls and the foxC1a MO-injected embryos. (B) Frontal/dorsal views of 22s hybridized embryos showing no differences in the expression of foxC1a and twist1a in migrating POM (arrowheads) between the controls and the nlz1 MO-injected embryos. (C and D) Lateral views of eyes from 31- to 32-hpf hybridized controls or embryos injected with the indicated MOs. (C) foxC1a, pitx2, and lmx1b1 expression in anterior POM (arrowheads) and/or within the choroid fissure (white triangles) is not affected in the nlz1 MO-injected embryos. (D) Expression of foxC1a, nlz1, and eya2 in anterior POM (arrowheads) is not affected in the pitx2 MO-injected embryos. (E) Lateral views of eyes from 32-hpf hybridized controls or noi embryos showing no appreciable differences in the expression of foxC1a, nlz1, pitx2, and lmx1b1 in anterior POM (arrowheads) and/ or within the choroid fissure (white triangles). |
|
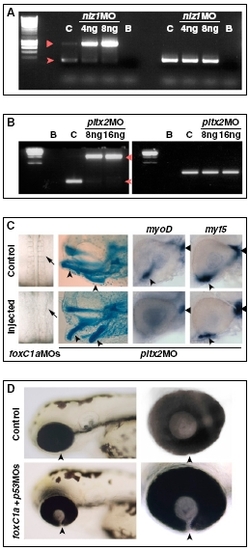
Controls for the activity of MOs against POM genes. (A) RT-PCR on RNA from 30-hpf controls (C) or embryos injected with 4 ng (M4) or 8 ng (M8) of nlz1 MO, showing disrupted splicing of nlz1 mRNA (left lanes). Wild-type RNA is nearly absent in nlz1 morphants and replaced by immature mRNA retaining the first intron, which is predicted to contain an in-frame stop codon after 39 bp. The arrowhead and triangle point to the spliced and nonspliced nlz1 PCR products, respectively. EF1α (right lanes) was used as a loading control. B, blank reactions. (B) RT-PCR on RNA from 30-hpf controls (C) or embryos injected with 8 ng (M8) or 16 ng (M16) of pitx2 MO, showing disrupted splicing of pitx2 mRNA (left lanes). Wild-type RNA was nearly absent in pitx2 morphants and replaced by immature mRNA retaining the second intron, which is predicted to contain an in-frame stop codon after 30 bp, leading to a truncated protein lacking the last 14 aa in the homeodomain and the whole C-terminal region. The arrowhead and triangle point to the spliced and nonspliced pitx2 PCR products, respectively. EF1α (right lanes) was used as a loading control. B, blank reactions. (C Left) Disrupted somitogenesis in a 18s embryos injected with foxC1a MOs. (Center) Abnormal visceral arch morphogenesis in a 4-d-old embryo injected with pitx2 MO, as shown by cartilage staining (arrowheads point to derivatives of the first two arches). (Right) Eyes of 32-hpf hybridized controls or pitx2 MO-injected embryos showing reduction of myoD-positive ventral extraocular muscles (arrowheads), but not dorsal myoD-positive muscles (triangles) or myf5-positive muscles, in the pitx2 morphants. (D) Coloboma in foxC1A morphants is not caused by apoptosis. Lateral views (Left) of the head region of 72-hpf control embryos or embryos that were injected with a combination of foxC1a MOs and p53 MO. The choroid fissure (arrowheads) is not closed in embryos coinjected with foxC1a MOs and p53 MO. (Right) Images are higher magnifications of the eyes. |