- Title
-
N-cadherin regulates primary motor axon growth and branching during zebrafish embryonic development
- Authors
- Bruses, J.L.
- Source
- Full text @ J. Comp. Neurol.
|
N-cadherin expression in primary motor neurons and muscle pioneer cells. A: Western blot analysis of anti-N-cadherin antibodies (see Table 1). Zebrafish (72 hpf) homogenates (40 μg of total protein/lane) were electrophoresed in a 10% SDS-polyacrylamide gel, electrotransferred to a PVDF membrane, and immunoblotted with a rabbit polyclonal anti-zebrafish N-cadherin (lane 1) and MNCD2 monoclonal anti-mouse N-cadherin (lane 2) antibodies. A single band of <120 kDa was detected in lane 1 and lane 2 by the rabbit polyclonal and MNCD2 antibodies, respectively (arrowhead). B: CHO cells were transfected with a plasmid expressing Gal4 under a CMV promoter and a plasmid carrying zebrafish N-cadherin and pren-EGFP under a 14X-UAS element (B1,2) or a plasmid expressing pren-EGFP under a 14X-UAS (B3,4). Cells were fixed and immunostained with anti-N-cadherin MNCD2 antibodies and anti-rat IgG Cy3-conjugated secondary antibodies. B1,2: Confocal images of the same cells showing expression of EGFP (B1) and N-cadherin (B2). B3,4: Confocal images of the same cells showing expression of EGFP (B3) while no N-cadherin labeling is detected (B4). Arrowheads point to the cell membrane, and asterisks indicate the perinuclear region. C,D: Neural tube of 24 hpf Tg(mnx1:GFP) embryos immunostained with anti-N-cadherin MNCD2 antibodies and observed under confocal microscopy. Arrowheads point to primary motor neuron cell bodies labeled with EGFP and expressing N-cadherin on the cell surface. E,F: Wild-type zebrafish embryos (24 hpf) doubly labeled with anti-N-cadherin MNCD2 antibodies (E) and with α-bungarotoxin (α-BTX) conjugated with Alexa 488 to detect nicotinic acetylcholine receptors (F). Brackets indicate the muscle pioneer cells at the horizontal myoseptum expressing N-cadherin (E) and a distinct cluster of acetylcholine receptors (F). G: Electron micrograph of a neuromuscular junction at the horizontal myoseptum from a 24 hpf wild-type zebrafish embryo. The arrowhead points to the synaptic cleft and the arrow to an active zone. H: Electron micrograph of a neuromuscular junction from a 120 hpf wild-type zebrafish larva. The arrowhead points to the synaptic cleft containing a characteristic basal lamina and the arrow points to an active zone determined by the presence synaptic vesicles fused to the presynaptic membrane. NT, neural tube; ST, synaptic terminal. In C–F, rostral is to the left and dorsal is to the top. Scale bars = 5 μm in B; 10 μm in C–F; 0.5 μm in G,H. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.] |
|
Analysis of cdh2hi3644Tg N-cadherin mutant zebrafish embryos. A,D: Photographs of 24 hpf wild-type zebrafish embryos. The arrowhead in D points to the midbrain–hindbrain boundary. B,E: 24 hpf cdh2hi3644Tg homozygote mutant embryos show defective tails (B, arrowhead) and disruption of the midbrain–hindbrain boundary (E, arrowhead). C,F: 24 hpf zebrafish embryos injected at the one-cell stage with antisense N-cadherin morpholinos (cdh2-MO-UTR). Knock-down of N-cadherin expression causes defective tail morphogenesis (C, arrowhead) and loss of the midbrain–hindbrain boundary (F, arrowhead). G: Genotyping of cdh2hi3644Tg embryos: +/+, wild type; forward and reverse primers anneal to N-cadherin (603 bp); –/–, homozygotes; forward primer anneals to the viral sequence and reverse primer anneals to N-cadherin sequence (415 bp); –/+, heterozygotes; both bands are detected. H: Immunoblot analysis of N-cadherin expression in tissue homogenates from 24 hpf embryos obtained from a cross of cdh2hi3644Tg heterozygous mutants. Embryos were genotyped and pooled as wild type (+/+), heterozygote (+/–), and homozygote (–/–), and homogenates were electrophoresed in a 10% SDS-polyacrylamide gel, transferred to a PVDF membrane, immunoblotted with MNCD2 antibodies, and detected by chemiluminescence. MNCD2 antibodies detected two bands of <105 kDa and <120 kDa. Densitometric analysis was used to quantify the relative amount of N-cadherin in each sample, which is expressed as percentage of wild type: +/+, wild type 100%; +/–, heterozygotes 64%; and –/–, homozygotes 22%. I: Immunoblot analysis with MNCD2 antibodies of N-cadherin expression in 96 hpf wild-type zebrafish larvae injected at the one-cell stage with antisense morpholinos against N-cadherin UTR (MO-UTR). Embryos injected with an N-cadherin antisense mismatched morpholino (MO-MIS) were used as control. MNCD2 detected two bands of <105 kDa and –120 kDa. J: Western blot of the same samples electrophoresed in I and immunoblotted with anti-E-cadherin antibodies revealed two bands of <120 kDa and <140k Da. E-cadherin protein expression levels were not affected by injection with N-cadherin antisense morpholinos. Scale bars = 0.1 mm. |
|
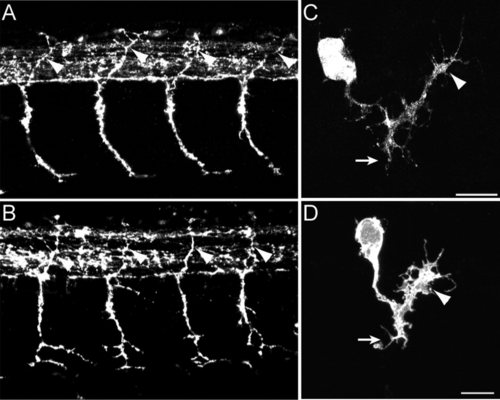
Loss of N-cadherin expression affects primary motor axon branching. A–H: Lateral views of 24 hpf zebrafish embryos immunostained with SV2 and znp1 antibodies, and observed via confocal microscopy. A,B: Primary motor axons in wild-type embryos exited the spinal cord and grew ventrally toward the horizontal myoseptum. The axons are enlarged at the area of contact with the muscle pioneer cells (brackets in B,D). C,D: Zebrafish embryos injected with N-cadherin antisense mismatched morpholinos (cdh2-MO-MIS) display axonal morphologies similarly to wild-type embryos. E,F: Embryos injected with antisense N-cadherin morpholino (cdh2-MO-UTR) reached the horizontal myoseptum but grew aberrant branches at the choice point (brackets) and in the ventral myotome (arrowheads). An axon branch is an extension of the axon from the center of the axon shaft >3 µm long. G,H: In cdh2hi3644Tg homozygous embryos, motor axons formed aberrant branches at the choice point (brackets) and ventral to the horizontal myoseptum (arrowheads). I: Analysis of the number of branches per 10 µm of axon expressed as mean ± SE [wild-type n = 27; cdh2-MO-MIS n = 29; cdh2-MO-UTR n = 26; cdh2hi3644Tg n = 26 (n, number of axons analyzed)]. J: Analysis of the average length of the branches expressed as mean ± SE [wild type n = 21; cdh2-MO-MIS n = 27; cdh2-MO-UTR n = 51; cdh2hi3644Tg n = 47 (n, number of branches examined)]. K: Analysis of the presynaptic surface area at the choice point expressed as mean ± SE [wild type n = 48; cdh2-MO-MIS n = 44; cdh2-MO-UTR n = 31; cdh2hi3644Tg n = 29 (n, number of axons examined)]. t-Test comparison of mutant and morpholino injected embryos vs. wild-type samples, *P < 0.05, **P < 0.005. WT, wild type. Rostral is to the left and dorsal is to the top. Scale bars = 10 μm. |
|
N-cadherin cdh2hi3644Tg mutants form higher numbers of neuromuscular junctions. Confocal images obtained from 120 hpf wild-type (A–C) and cdh2hi3644Tg (D–F) larvae doubly labeled with SV2 antibodies (A,D) and anti-mouse IgG Cy3-conjugated secondary antibody and with α-bungarotoxin (α-BTX) conjugated to Alexa 488 (B,E). Approximately 30-μm-thick stacks of confocal images obtained at 1-μm intervals were projected to a single plane. C,F: Merged images from A,B and D,E, respectively. Arrowheads point to the somitic myoseptum. G,H: High-magnification images of the boxed areas in C and F, respectively. I–K: Analysis of the number (I) and size (J) of SV2 and α-BTX clusters and of the percentage of colocalization between pre- and postsynaptic markers (K). Three confocal sections at the central region of the myotome were rendered to a single plane and used for quantification of the SV2 and α-BTX clusters. Open bars, wild type (WT; n = 16); gray bars, cdh2hi3644Tg (hi3644; n = 27; n, number of somitic hemisegments analyzed). Asterisks in I indicate a Student′s t-test P value < 0.005. Rostral is to the left and dorsal is to the top. Scale bars = 10 μm in A (applies to A–C); 10 μm in D (applies to D–F); 10 μm in G,H. |
|
MiP motor axon targeting is unaffected in N-cadherin mutant embryos. Wild-type (A) and cdh2hi3644Tg mutant (B) embryos were fixed at 27 hpf, immunolabeled with znp1 antibodies, and observed via confocal microscopy. Arrowheads point to the presumptive MiP axon in the dorsal myotome. The axons appear to have followed a distorted pattern of migration. C,D: Single MiP motor neurons labeled by mosaic expression of pren-EGFP using a mix of a plasmid expressing Gal4 under the mnx1 promoter and a plasmid expressing pren-EGFP under a 14X-UAS element. In all cases, the motor axons reached the horizontal myoseptum and extended a dorsal branch. Wild type, n = 6; cdh2hi3644Tg mutant, n = 6. Rostral is to the left and dorsal is to the top. Scale bars = 10 μm. |
|
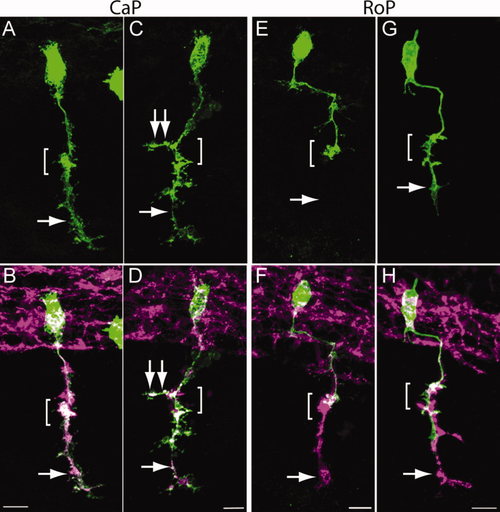
CaP and RoP motor axons grow abnormal branches in N-cadherin mutant embryos. Wild-type and cdh2hi3644Tg embryos were injected at the one-cell stage with a mix of two plasmids expressing Gal4 under the mnx1 promoter and pren-EGFP under a 14X-UAS element, fixed at 24 hpf, immunolabeled with SV2 and znp1 antibodies, and observed via confocal microscopy. A,C,E,G show pren-EGFP labeling; B,D,F,H show pren-EGFP (green) merged with SV2 and znp1 antibody labeling. A,B: Wild-type CaP motor neuron shows the characteristic morphology, with an axon extending from the spinal cord along the common pathway and into the myotome (arrow) ventral to the choice point (bracket). C,D: CaP motor neuron from a cdh2hi3644Tg embryo. The bracket indicates the choice point; the single arrow points to the axon in the ventral myotome; the double arrows point to an aberrant branch extending in the rostrocaudal axis. E,F: Wild-type RoP motor axon shows the characteristic caudal migration before turning ventrally into the myotome. The bracket indicates the choice point where the RoP axon normally stalled. The SV2 and znp1 labeling ventral to the choice point corresponds to the CaP motor axon. G,H: RoP motor neuron from a cdh2hi3644Tg mutant embryo. The bracket indicates the choice point, and the arrow points to aberrant growth of the axon into the ventral myotome together with the CaP motor axon. Rostral is to the left and dorsal is to the top. Scale bars = 10 μm in B (applies to A,B); 10 μm in D (applies to C,D); 10 μm in F (applies to E,F); 10 µm in H (applies to G,H). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.] |
|
Tg(mnx1:Gal4-VP16) embryos were injected at the one-cell stage with a plasmid encoding pren-EGFP under a 14X-UAS element, fixed at 24 hpf, and immunostained with SV2 and znp1 antibodies. Arrowheads point to primary motor neurons expressing EGFP. B,D: Higher magnification images of the boxed areas in A,C, respectively. Arrowheads point to primary motor neuron somas. E–I: Tg(mnx1:Gal4-VP16) embryos were injected at the one-cell stage with the 14X-UAS-IL2α-EGFP plasmid (E,F) or with 14X-UAS-IL2α-CD and pren-EGFP plasmid (G–I). Embryos were fixed at 24 hpf and immunostained with anti-IL2α antibodies (F,H,I). IL2α-EGFP is evenly distributed throughout the motor neuron (E,F). Pren-EGFP labels the entire cell body and axon (G), whereas IL2α-CD is detected as discrete puncta through out the cell (H, arrowheads). I: Higher magnification of the axon shown in G, indicating IL2α-CD puncta accumulated at the choice point (bracket) and at the distal tip of the axon. Dorsal is to the top and rostral is to the left. Scale bars = 50 μm in C (applies to A,C); 10 µm in D (applies to B,D); 10 μm in F (applies to E,F); 10 μm in H (applies to G,H). |
|
Expression of N-cadherin dominant-interfering cytoplasmic domain perturbs CaP motor axon growth at the horizontal myoseptum. A–I: Tg(mnx1:Gal4-VP16) embryos were injected at the one-cell stage with IL2α-EGFP (A–C) or with IL2α-cdh2-CD and pren-EGFP (D–I) plasmids. Embryos were fixed at 24 hpf, immunostained with SV2 and znp1 and observed under confocal microscopy. A–C: Arrow points to the CaP axon extending ventral to the horizontal myoseptum (HMS), indicated with a dashed line. The IL2α-EGFP labeled axon has a normal morphology compared with the untransfected neighboring axons labeled with SV2 and znp1. D–F: Motor axon expressing IL2α-cdh2-CD grew through the common pathway but stalled at the horizontal myoseptum. The arrow points to the absence of SV2- and znp1-labeled CaP axon ventral to the choice point, whereas neighboring untransfected axons grew normally. G–I: CaP motor neuron expressing IL2α-cdh2-CD and pren-EGFP in which the axon extended ventrally to the horizontal myoseptum but showed a shorter migration distance compared with an untransfected SV2- and znp1-labeled axon (arrow). Dorsal is to the top and rostral is to the left. Scale bars = 10 μm in C (applies to A–C); 10 μm in F (applies to D–F); 10 μm in I (applies to G–I). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.] |