- Title
-
Evolutionarily conserved function of Gbx2 in anterior hindbrain development
- Authors
- Burroughs-Garcia, J., Sittaramane, V., Chandrasekhar, A., and Waters, S.T.
- Source
- Full text @ Dev. Dyn.
|
gbx2 morpholino decreases the distance between midbrain and r3 and disrupts cranial nerve V motor neuron organization. A–F: Dorsal views of wild-type and gbx2 morphant anterior hindbrains. A,B: Two-color in situ hybridization examining the expression of hoxa2 (blue) and krox20 (red) at 10-somite stage. C,D: Two-color in situ hybridization examining the expression of pax2.1 (black) and krox20 (red) at 10-somite stage. Anterior hindbrain truncation between posterior midbrain (pax2.1) and r3 (krox20) is visible in embryos injected with 8 ng gbx2-MO at 10-somites (D). E,F: Two-color in situ hybridization examining the expression of pax2.1 (black) and hoxa2 (purple) at 10-somite stage. G,H: Anti-GFP antibody staining at 36 hpf. Embryos that were injected with 8 ng of gbx2-MO show abnormal clustering of nV cell bodies clustering in r2 and r3 (arrowhead in H), and ectopic axonal projections (arrow in H) The migration and organization of nVII motor neurons in r4–r7 are not affected (G,H). I: Distance between posterior midbrain and r3. J: Rhombomere width. *Midbrain (nIII and nIV) motor neurons. **P < 0.0001 (Student′s t-test). hpf, hours post fertilization; MO, morpholino; r, rhombomere; s, somite stage. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Expression of mid-hindbrain markers is not affected in gbx2 morphants. A–L: Comparative analysis of gbx1 expression in wild-type embryos (A,C,E,G) and embryos injected with 8 ng of gbx2-MO (B,D,F,H). A,B: Dorsal views with anterior to the top. C–F: Lateral views with anterior to the left. G,H: Dorsal views wth anterior to the left. I–L: Comparative analysis of fgf8 expression in wild-type (I,K) and gbx2 morphants (J,L). ov, otic vesicle; 80%, 80% epiboly; s, somite-stage; *, midbrain/hindbrain boundary. EXPRESSION / LABELING:
|
|
Reduced proliferation in gbx2 morphant embryos. A–F: Embryos were labeled with the anti-pH3 antibody to label cells undergoing mitosis. A,B: Lateral views of uninjected and embryo injected with 8 ng of gbx2-MO illustrates that the decrease in mitotic figures occurs at 10-somites. Inset (A–D): Transverse section demonstrates mitotic figures in the neural tube of the r2 domain (arrow). C,D: Lateral views at 20-somite stage shows that a decrease in pH3-positive cells persists in gbx2 morphant embryos. E: Number of pH3-positive cells in the r2 domain of the anterior hindbrain as indicated in the insets. F: Number of pH3-positive cells in the forebrain. Brackets, anterior hindbrain; MO, morpholino; s, somite stage. PHENOTYPE:
|
|
Injection of gbx2 morpholino induces anterior hindbrain cell death. A–Q: Effect of gbx2-MO, p53-MO, and gbx2-MO/p53-MO co-injection on embryos. A–H: Lateral view, anterior to the left of embryos stained with acridine orange and viewed by fluorescent microscopy at 10-somite (A–D), 20-somite (E–H), and 24 hpf (inset in E–H) showing increased cell death in r2–r5 at 20-somites and r2, r3, and r5 at 24 hpf in gbx2 morphants. I–P: Dorsal views, with anterior to the left. I–L: Two-color in situ hybridization examining the expression of pax2.1 (black) and krox20 (red) at 20-somite stage, shows anterior hindbrain truncation in gbx2 morphants. L: Co-injection of gbx2-MO/p53-MO slightly reduces anterior hindbrain truncation. M–P: GFP DAB antibody staining of 36-hpf embryos examining cell body clusters. Q: Distance between posterior midbrain and r3. Arrows indicate disruption of distinct nV clustering in r2 and r3. *, Position of r4; **P < 0.0001 (Student′s t-test); hpf, hours post-fertilization; MO, morpholino; r, rhombomere; s, somite stage. EXPRESSION / LABELING:
PHENOTYPE:
|
|
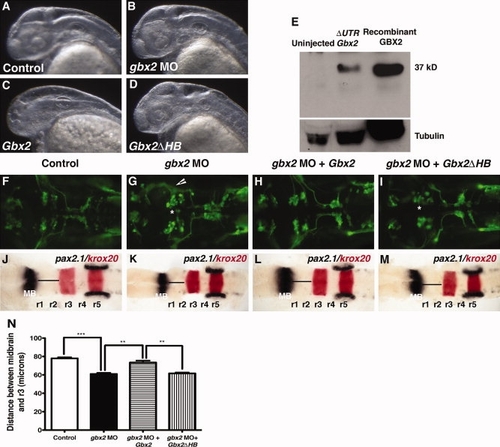
Mouse GBX2 functions in Zebrafish. Overexpression of mouse Gbx2 mRNA affects the formation of the forebrain in zebrafish embryos. A–D: Lateral view at 36 hpf, anterior to the left of embryos viewed by darkfield microscopy. A,B,D: Control embryo (A) and embryos injected with 8 ng gbx2-MO (B) or 120 pg of truncated mouse Gbx2 mRNA (D) do not show morphological defects in the forebrain. C: Partial or complete truncation of the forebrain was observed in embryos injected with complete mouse Gbx2 coding sequence. E: Western blot of total lysates from 10-hpf embryos showing expression of mouse GBX2 protein translated from complete ΔUTRGbx2. Recombinant mouse GBX2 protein expressed in E. coli, was used as a positive control. Expression of mouse GBX2 rescues anterior hindbrain defects caused by gbx2-MO. F–M: Dorsal views anterior to the left of Tg(isl1:gfp) embryos. F–I: The expression of GFP at 36 hpf. Abnormal cell body clustering was observed in embryos injected with gbx2-MO (B). J–M: Whole-mount in situ hybridization examining the expression of pax2.1 (black) and krox20 (red) at 20-somite stage. K: Injection of gbx2-MO results in truncation between the midbrain and r3. Embryos co-injected with gbx2-MO/Gbx2 mRNAs show complete rescue of abnormal nV cell body clustering (H) and anterior hindbrain truncation (L). Co-injection with gbx2-MO/Gbx2ΔHB mRNAs does not rescue anterior hindbrain defects (I,M). N: Distance between posterior midbrain and r3. Arrows indicate disruption of distinct nV clustering in r2 and r3. *, Abnormal nV cell body clustering. **P < 0.001 (Student′s t-test), ***P < 0.0001 (Student′s t-test); arrowheads, ectopic axonal branching; hpf, hours post fertilization; HB, homeobox; MB, midbrain; MO, morpholino; r, rhombomere. PHENOTYPE:
|
|
Control morpholino does not result in anterior hindbrain phenotypes. A–D: Dorsal view of uninjected and embryos injected with the control morpholino. A,B: No anterior hindbrain truncation occurs in embryos injected with control-MO. C,D: nV cell body clustering is normal in embryos injected with control-MO. |
|
Expression of GBX2ΔHB in injected embryos. A, B: Western blot of total lysates from 10 hpf embryos showing expression of mouse GBX2ΔHB protein translated from Gbx2ΔHB mRNA. Recombinant mouse V5- GBX2ΔHB fusion protein expressed in E. coli was used as a positive control. A1–3: Detection of GBX2ΔHB using chicken anti-GBX2 in (1) lysates from uninjected embryos, (2) lysates from embryos injected with 120 pg Gbx2ΔHB mRNA, (3) recombinant mouse V5- GBX2ΔHB fusion protein. A4: Detection of recombinant mouse V5-GBX2ΔHB fusion protein using anti-V5 antibody. B1–3: Detection of Myc-tagged GBX2ΔHB using anti-Myc (1) lysates from uninjected embryos, (2) lysates from embryos injected with 240 pg Gbx2ΔHB mRNA, (3) lysates from embryos injected with 120 pg Gbx2ΔHB mRNA. C: Tubulin loading control. |