- Title
-
Wnt/β-catenin dependent cell proliferation underlies segmented lateral line morphogenesis
- Authors
- Aman, A., Nguyen, M., and Piotrowski, T.
- Source
- Full text @ Dev. Biol.
|
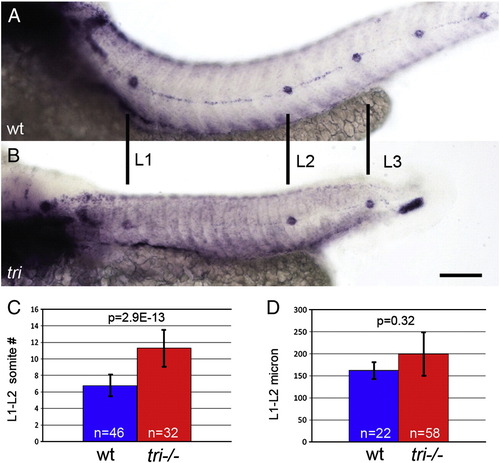
Proneuromast deposition is not triggered by traversing somite boundaries. (A,B) eya1 in situ hybridization labels deposited proneuromasts. (A) A wt embryo with a typical distribution of proneuromasts. (B) A tri homozygous mutant displays similarly spaced proneuromasts, even though somites are narrower. (C) Quantification of somites between the first two proneuromasts in wt and tri mutants. Mutant primordia cross significantly more somite boundaries between proneuromast depositions in tri mutants. (D) The actual distance between these proneuromasts in wt and tri mutants is not significantly different. Scale bar equals 50 μm. Error bars represent standard deviation from the mean. EXPRESSION / LABELING:
|
|
Increasing apoptosis leads to deposition of fewer proneuromast (A,B) PH3 positive cells are labeled green. (D,E,G,I,K,M) Primary lateral line morphology revealed by klf4 in situ hybridization. Proneuromasts are numbered and the terminal proneuromasts are labeled with a ‘T’. Presence of terminal proneuromasts demonstrates completed primordium migration. (A,C) Primordia treated with DMSO from 26 to 32 hpf proliferate normally. (D) DMSO treated primordia migrate and deposit a normal complement of proneuromasts along the trunk. (B,C) Treatment with 75 μM aphidicolin + 7.5 mM hydroxyurea (HUA) from 26 to 32 hpf causes a reduction in the proportion of mitotic cells expressing PH3. (E) HUA treatment from 26 to 54 hpf leads to a significant reduction in the number of deposited proneuromasts without preventing primordium migration. (F,H,J,L) Whole mount TUNEL assay at 32 hpf. Nuclei are labeled with DAPI and red spots represent TUNEL signal. Dying cells are rapidly extruded and form a cloud surrounding and trailing behind the migrating primordium. (F) No appreciable apoptosis is detectable in a wt primordium. (G) A typical 48 hpf wt primary lateral line. (H) bap28 homozygous mutants show a dramatic increase in apoptosis. (I) bap28 mutants possess fewer primary proneuromasts but still produce terminal proneuromasts. (J) Injection of toxic tcf7 ATG MO leads to apoptosis in the primordium. (K) tcf7 ATG MO also causes deposition of fewer proneuromasts, though to a lesser extent than bap28 mutants. (L) Maternal/zygotic tcf7 null mutants show no apoptosis in the primordium. (M) tcf7 mutants have a normal number of primary proneuromasts. (N) Quantification of proneuromast number. Manipulations that decrease the rate of cell proliferation or increase apoptosis lead to deposition of fewer proneuromasts. This effect of apoptosis can be rescued by co-injection of p53 MO. Error bars equal standard deviation. Scale bars equal 50 μm. |
|
Slowing cell addition does not effect primordium migration, proneuromast deposition or proliferation (A,B) primordia (within white ovals) and proneuromasts (within white rectangles) stained with DAPI (grey) at 32 hpf. (A) Wt primordium. (B) tcf7 morphant primordium. (C) Somite positions of the leading edge of control primordia (blue) and tcf7 morphant or HUA treated primordia (red) at 32 hpf are not significantly different, demonstrating that migration speed is normal in injected embryos. (D). Control and tcf7 morphant or HUA treated primordia deposit proneuromasts that are not significantly different in cell number. TUNEL staining in DMSO treated primordia (E) and HUA treated primordia (F) shows that this treatment does not cause apoptosis within the primordium. (G) BrdU labeling (red) in wt and (H) bap28 mutant primordia, counterstained with DAPI (grey). (I) The BrdU indexes of wt and bap28 mutant primordia are not significantly different. |
|
Primordium lengthening precedes proneuromast deposition. Still images of timelapse movies of proneuromast deposition in control and tcf7 MO injected Tg(claudinb:GFP) transgenic embryos (A–H). (A–D) Wt primordia in different phases of the deposition cycle. (A) Wt primordium immediately prior to deposition of the first primary proneuromast (L1). (B) The same primordium immediately following deposition of L1. (C) Wt primordium preceding deposition of the second proneuromast (L2). (D) Wt primordium following deposition of L2. (E,F) tcf7 morphant primordia before deposition and following deposition of L1. (G,H) The same primordium immediately before and following deposition of L2. (I) Quantification of primordium lengths in wt embryos (blue, n = 4) and tcf7 morphants (red, n = 7). Wt and tcf7 morphant primordia achieve similar lengths preceding deposition of L1 (A,E,I). Wt and tcf7 morphant primordia shrink to similar sizes following deposition of L1 (B,F,I). tcf7 morphant primordia may be slightly shorter than wt primordia preceding L2 deposition (C,G,I). However, following deposition of L2, wt and tcf7 morphant primordia again possess the same length (D,H,I). EXPRESSION / LABELING:
|
|
cxcr7b expression begins at a constant distance from the leading edge. (A,B) cxcr7b in situ hybridization labels the trailing portion of the primordium. (A) Example of a long primordium that is about to deposit a proneuromast. Such long primordia possess a relatively wide cxcr7b expression domain. (B) A primordium that has just deposited a proneuromast. Most of the cxcr7b expressing cells were deposited yielding a short primordium with a relatively narrow cxcr7b expression domain. Total length (green), length of the cxcr7b expression domain (blue), and the length of the cxcr7b-free domain (red) were measured in primordia during various phases of the deposition cycle (n = 8). (C) The length of the cxcr7b expression domain fluctuates with primordium length. Longer primordia about to deposit have longer cxcr7b expression domains (slope = 0.9; R2 = 0.80). (D) The length of the cxcr7b free zone remains relatively constant as primordium size fluctuates (slope = 0.1). Scale bar equals 50 μm. EXPRESSION / LABELING:
|
|
Wnt/β-catenin and Fgf signaling synergistically control proliferation in the primordium. (A,G,J,M,P) BrdU labeling in the primordium (red). Embryos were given a one hour pulse of BrdU at 32 hpf and immediately fixed. Nuclei are stained with DAPI (grey). (B,E,H,K,N,Q) Schematic representations of the distribution of Wnt/β-catenin signaling (red), Fgf signaling (green), and the region of overlap between these pathways (yellow). (C,F,I,L,O,R) Quantification of BrdU indexes for wt, Tg(hs:Dkk1), apc and SU5402 treated primordia. (A) Wt primordium with the leading zone and trailing rosette marked. Note the relatively low level of BrdU in the trailing rosette. (B) Schematic representation of Wnt/β-catenin and Fgf signaling activity in pre-deposition wt primordia. (C) Quantification of proliferation in the leading zone and trailing rosette of pre-deposition wt primordia. BrdU index is higher in the leading zone of wt primordia relative to the trailing rosette. (D) Schematic representation of a pre-deposition primordium harboring three rosettes (red) and a post-deposition primordium harboring two rosettes (blue). (E) Post-deposition primordia are shorter than pre-deposition primordial and have a smaller region free of wnt/β-catenin signaling. (F) Post-deposition primordial have a significantly higher BrdU index compared to pre-deposition primordia due to the loss of the relatively quiescent trailing rosette. (G) BrdU in a Tg(hs:Dkk1) primordium. Embryos were heatshocked six hours prior to BrdU incorporation. (H) Induction of Dkk1 leads to loss of Wnt/β-catenin and Fgf signaling in primordium. (I) Induction of Dkk1 significantly reduced BrdU. (J) apc mutant embryo. BrdU labeling is homogeneous throughout the mutant primordium. (K) apc mutation leads to the activation of both the Wnt/β-catenin and Fgf pathways throughout the primordium. (L) apc mutant primordial have a significantly higher BrdU index compared to wt sibs. The BrdU index in apc mutant primordia is similar to the index in the leading zone of wt primordia (compare (A) and (J)). (M) An embryo treated with 5 μM SU5402 from 22 to 32 hpf. (N) In SU5402 treated embryos Wnt/β-catenin signaling activity expands throughout the primordium. (O) The Fgf inhibitor SU5402 significantly reduces the BrdU index. (P) Induction of constitutively active Fgf receptor by heatshocking Tg(hs:ca-fgfr1) embryos reduces BrdU incorporation in the primordium. (Q) ca-fgfr1 expression leads to increased stimulation of Fgf signaling and a subsequent loss of Wnt/β-catenin signaling. (R) cafgfr1 expression leads to a significant reduction in BrdU incorporation. |
|
Epithelial rosette formation does not correlate with the proliferative behavior of cells. (A–C′) Labeling of the epithelial adherens junction protein ZO1 (red) in Tg(Claudinb-GFP) embryos. (A′–C′) ZO-1 labeling in the primordium. (A, A′) In wt primordia, epithelial rosette formation involves the apical constriction of cells within the primordium and the accumulation of apical adhesion complexes containing ZO-1 (arrows). Proliferation occurs in the unpatterned leading zone and immature rosettes. Cells in the most mature trailing rosette hardly proliferate. (B, B′) Inhibiting Fgf signaling using SU5402 causes a loss of apical constriction and ZO-1 accumulation in addition to a strong loss of proliferation. This finding argues that epithelial rosettes are not the cause of reduced proliferation in the trailing zone of wt primordia. (C, C′) in apc mutants, characterized by constitutively active Wnt/β-catenin and Fgf signaling in the primordium, apical constriction and ZO-1 accumulation occurs similarly as in wt primordia. In contrast to wt primordia, in apc mutants the trailing rosette is characterized by a high level of proliferation. Scale bars represent 20 μM. EXPRESSION / LABELING:
|
|
hmx2 and hmx3 are required but not sufficient to induce proliferation in the primordium. (A) In wt embryos hmx2 is expressed broadly in the leading portion of the primordium but is reduced in the trailing region. hmx3 expression is identical (not shown). (B) In apc mutant embryos, in which the Fgf and the Wnt/β-catenin pathways are upregulated, hmx2 is uniformly expressed in all cells of the primordium suggesting that these genes might be regulated by these pathways. (C) Reducing Wnt/β-catenin signaling by heatshock induction of dkk1 in Tg(hs:Dkk1) embryos leads to a downregulation of hmx2 demonstrating that Wnt/β-catenin signaling is an important regulator of hmx2 expression. (D) On the other hand, treatment with the Fgfr1 inhibitor SU5402 revealed that the Fgf pathway does not regulate hmx2 and hmx3 expression, as previously described (Feng and Xu, 2010). In SU5402 treated embryos hmx2 expression is ubiquitously strong, as Wnt/β-catenin is upregulated in these primordia. Even though hmx2 is expressed, SU5402 treated primordia do not proliferate demonstrating that hmx2 and hmx3 are not sufficient to induce proliferation in the absence of other Fgf dependent factors. EXPRESSION / LABELING:
|
|
Reducing Fgf signaling in heatshocked Tg(hs:DNfgfR1) primordia leads to a reduced BrdU index. BrdU labeling (red) in heatshocked, non-transgenic sibs (A) and heatshocked Tg(hs:DNfgfR1) primordia (B), counterstained with DAPI (grey). Embryos were heatshocked at 26 hpf, six hours prior to receiving a one hour BrdU pulse at 32 hpf and immediately fixed. (C) Reducing Fgf signaling by heatshocking Tg(hs:DNfgfR1) embryos leads to a significant reduction in BrdU incorporation (BrdU index for heatshocked, non-transgenic sibs = 0.28 ± 0.10, n = 18; BrdU index for heatshocked tg(hs:DNfgfR1) = 0.21 ± 0.10; n = 16; Student′s t-test p = 0.005). |
|
Heatshock induction of ca-fgfr1 stimulates Fgf signaling in the primordium. (A,B) Whole mount phospho-tyrosine antibody staining at 32 hpf, six hours post heatshock. Embryos were mounted and photographed together to accurately display intensity difference. (A) Non-transgenic sibs express phospho-tyrosine broadly. (B) Embryos harboring the Tg(hs:ca-fgfr1) transgene show much more intense phospho-tyrosine staining demonstrating that the transgene activates Fgf signaling throughout the animal. (C,D) In situ hybridization with the Wnt/β-catenin pathway target lef1 in the primordium at 32 hpf six hours post heatshock. (C) In 32 hpf non-transgenic sibs lef1 is expressed in the leading zone of the primordium. (D) Induction of ca-fgfr1 leads to a loss of Wnt/β-catenin signaling in the primordium. |
Reprinted from Developmental Biology, 349(2), Aman, A., Nguyen, M., and Piotrowski, T., Wnt/β-catenin dependent cell proliferation underlies segmented lateral line morphogenesis, 470-482, Copyright (2011) with permission from Elsevier. Full text @ Dev. Biol.