- Title
-
PRE-1, a cis element sufficient to enhance cone- and rod- specific expression in differentiating zebrafish photoreceptors
- Authors
- Morrissey, M.E., Shelton, S., Brockerhoff, S.E., Hurley, J.B., and Kennedy, B.N.
- Source
- Full text @ BMC Dev. Biol.
|
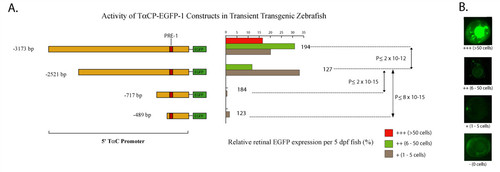
A ~0.5 kb 5′ promoter fragment of zebrafish TαC is sufficient to initiate photoreceptor specific expression in differentiating cones. A. Schematic of the promoter-reporter portion of the zebrafish TαC promoter constructs and graphs of their corresponding activity in transgenic zebrafish larvae at 5 dpf. The number of transgenic fish scored for each construct is indicated at the right of the histograms. B. Examples of transient transgenic zebrafish eyes showing the activity levels used to determine the activity of the constructs. |
|
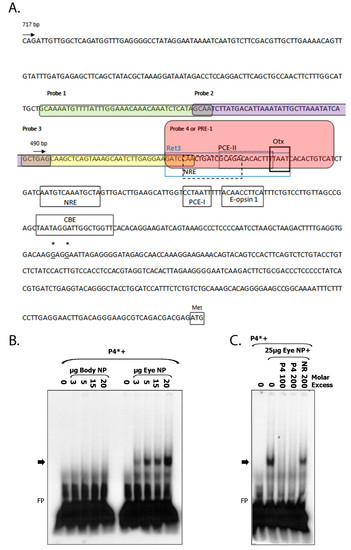
Identification of PRE-1 in TαCP. A. Annotated zebrafish TαC proximal promoter sequence. The sequence of probes 1-4 used in electrophoretic mobility shift assays are highlighted by rounded boxes. The PRE-1 (probe 4) sequence is highlighted in pink. The most 5′-end of zebrafish TαC ESTs (accession numbers DY550630, BG307391) are indicated by asterisks and underlined. The translation start codon is indicated by a box and methionine (Met). Putative trans-factor binding sites for Ret 3, NRE, PCE-1, PCE-II, Otx, CBE, Nr2e3 and E-boxes, based on homology to known cis-elements are indicated by boxes [26-29,33,36,51,52]. NRE, Nrl Response Element. PCE, photoreceptor conserved element. CBE, Crx-binding element. B. PRE-1 specifically binds nuclear factors expressed in ocular tissue. Electrophoretic mobility shift assay of radiolabelled PRE-1 (TαC probe 4) incubated with increasing concentrations of zebrafish eye or body nuclear protein. PRE-1 binds nuclear protein isolated from adult zebrafish eyes but not body nuclear protein. C. Competition analyses indicate that binding of trans-factor(s) is specific because unlabelled PRE-1 at 100 or 200-fold molar excess is able to compete away binding of eye nuclear protein to labelled PRE-1, whereas an unlabelled non-related competitor at 200-fold molar excess is not. Nuclear protein (NP), non-related (NR), probe 4 (P4 or PRE-1), free probe (FP). Asterisks indicate radiolabelled probe and arrow indicates the DNA-protein complex of interest. |
|
The majority of the 41 bp PRE-1 sequence is required for binding of nuclear factors in vitro. Large deletions and 3 bp mutations of PRE-1 eliminate trans-factor(s) binding. A. Electrophoretic mobility shift assay demonstrates that PRE-1 deletion probes P4.1-3 are unable to form a complex with eye nuclear protein but are sufficient to compete away factors from binding to full length P4 at the indicated molar excess of unlabelled probe. C. Electrophoretic mobility shift assay demonstrates that mutant PRE-1 probes P4MA-D are unable to bind eye nuclear protein but P4ME is, indicating that 3 bp sequences mutated in A-D are necessary for trans-factor(s) binding to PRE-1. B, D. Sequence of PRE-1 deletion and mutant probes. Nucleotides required for binding are indicated by boxes and 3 bp mutated sequences are in lowercase and underlined. Nuclear protein (NP), non-related (NR), probe 4 (P4 or PRE-1), free probe (FP). Asterisks indicate radiolabelled probe and arrow indicates the DNA-protein complex of interest. |
|
PRE-1 is sufficient to enhance reporter expression from photoreceptor-specific promoters. A. Schematic of the promoter-reporter regions of chimeric constructs. Three copies of PRE-1 (red boxes) are sufficient to enhance expression from both cone and rod promoter fragments, whereas three copies of PRE-1-rho (navy boxes) are unable to enhance expression from the cone promoter fragment. Blue and purple rectangles represent zebrafish UV opsin and rhodopsin promoter fragments respectively, upstream of EGFP. B-E. Confocal micrographs of retinal sections from 5 dpf embryos injected with 1.2 kb ZOP-EGFP or (3x PRE-1)-1.2 kb ZOP-EGFP constructs and stained with 4C12 and zpr-1 antibodies. EGFP-positive cells co localize (arrows) with rod photoreceptors (4C12 antibody), but not with cone photoreceptors (zpr-1 antibody). Nuclei are counter-stained with DAPI. |