- Title
-
Knockdown and overexpression of Unc-45b result in defective myofibril organization in skeletal muscles of zebrafish embryos
- Authors
- Bernick, E., Zhang, P., and Du, S.
- Source
- Full text @ BMC Cell Biol.
|
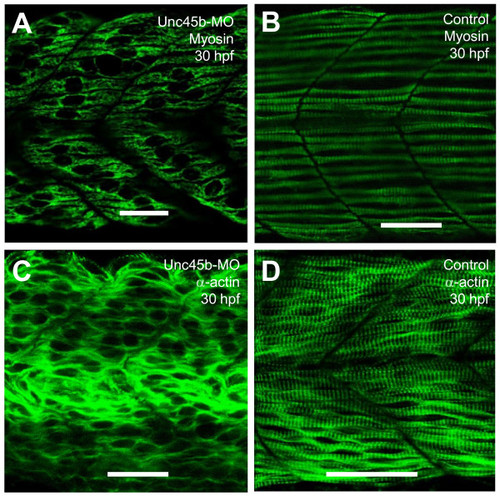
Effects of unc-45b knockdown on thick and thin filament organization in skeletal muscles of zebrafish embryos. A, B. Anti-MyHC antibody (F59) staining shows the organization of thick filaments in trunk slow muscles of uninjected control (B), or unc-45b knockdown (A) embryos at 30 hpf. C, D. Anti-α-actin antibody staining shows the organization of thin filaments in trunk muscles of uninjected control (D) or unc-45b knockdown (C) embryos at 30 hpf. Scale bar = 25 μm in A, B, C, D. |
|
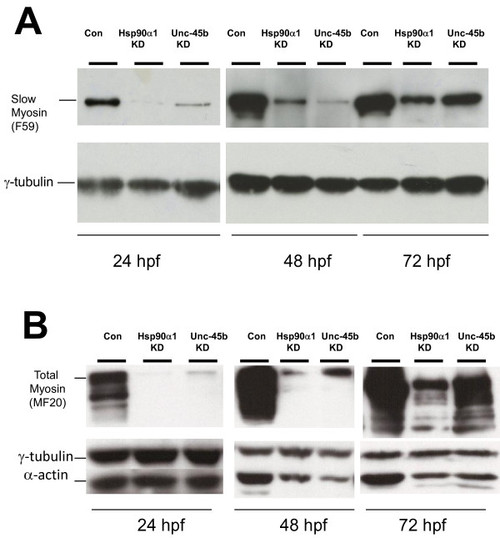
Knockdown of Unc-45b or Hsp90α1 results in significant reduction of myosin and α-actin accumulation. Western blot was performed using protein extracts from Unc-45b or Hsp90α1 knockdown, or uninjected control embryos at 24, 48 and 72 hpf using anti-myosin antibodies F59 (A) and MF20 (B) that recognize slow muscle myosin or general myosin proteins, respectively. The data show decreased levels of slow or total myosin proteins in Unc-45b and Hsp90α1 knockdown embryos at 24 and 48 hpf. A partial recovery of myosin expression was noted in 72 hpf embryos. Decreased levels of α-actin were also detected in the Unc-45b and Hsp90α1 knockdown embryos at 48 and 72 hpf. γ-tublin was used as loading control. Protein extract from five embryos each was used at 24 hpf. Protein extracts from three embryos were used at 48 and 72 hpf. |
|
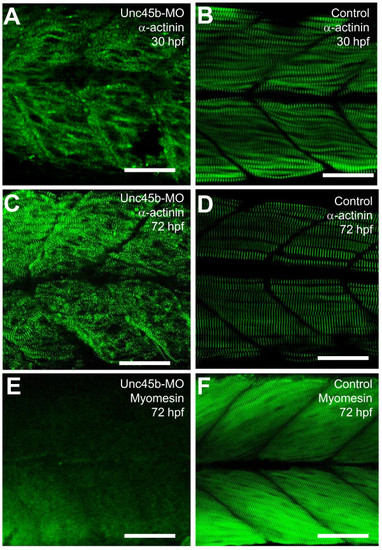
The effect of unc-45b knockdown on α-actinin and myomesin localization in skeletal muscles of zebrafish embryos. A-D. Anti-α-actinin antibody staining shows the Z-disc organization in unc-45b knockdown (A, C) or uninjecetd control (B, D) embryos at 30 (A, B) or 72 (C, D) hpf. E, F. Anti-myomesin antibody staining shows the M-line organization in unc-45b knockdown (E), or control (F) embryos at 72 hpf. Scale bars = 25 μm |
|
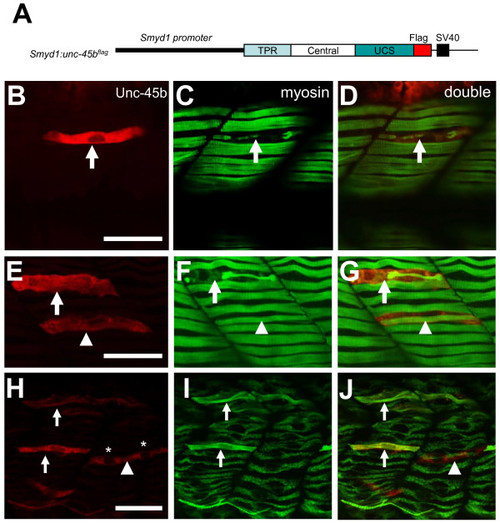
Overexpression of Unc-45b resulted in defective thick filament organization. A. The DNA construct expressing a flag-tagged Unc-45b under the control of smyd1 promoter was injected into zebrafish embryos. The effect on thick filament organization was analyzed by double antibody staining at 30 hpf. B-D. Double staining with anti-flag (red) and anti-myosin (F59, green) antibodies shows the expression of flag-tagged Unc-45b (B) in a single fiber and its effect on myosin thick filament organization (C). D represents the merged image of B and C showing that the myofibril defect is restricted to the myofiber expressing the flag-tagged Unc-45b. Fifty six myofibers from 40 embryos were analyzed. 70% of the myofiber showed the disorganized thick filaments. E-G. Double staining shows the expression of flag-tagged Unc-45b in two myofiber with different phenotypes on myosin thick filament organization. One myofiber exhibited disorganized thick filament (Arrow). In contrast, another myofiber exhibited normal thick filament organization (arrow head). However, this fiber appeared to be skinnier compared with its neighbors without the ectopic Unc-45b expression. H-J. Double staining shows the rescue of thick filament organization in Unc45b knockdown zebrafish embryos co-injected Unc45b-MO with the smyd1:unc45bflag DNA construct. Myofibers expressing the flag-tagged Unc45b (H) exhibited normal thick filament organization (I, J). The fast fiber with two nuclei (*) is indicated by the arrow head. F59 does not label myosin expressed in fast muscles. Thirty five slow myofibers from 29 embryos were analyzed. 100% of the slow myofiber expressing the flag-tagged Unc45b (H) exhibited normal thick filament organization. Scale bars = 15 μm. |
|
Deletion of the N-terminal TPR domain had no effect on the disruptive activity of Unc-45b on myosin thick filament organization, whereas deletion of the C-terminal UCS domain abolished the disruptive effect of Unc-45b overexpression. A. DNA constructs, smyd1:unc-45bΔTPRflag and smyd1:unc-45bΔUCSflag, were generated as minigenes expressing Unc-45b mutant proteins without the N-terminal TPR domain (amino acids 1-119), or the C-terminal UCS domain (amino acids 537-934). A flag-tag was fused in frame at the C-terminus for monitoring gene expression. The DNA construct was injected into zebrafish embryos. The effect on thick, thin filament and Z-line organization was analyzed by double staining using anti-flag (red) and anti-myosin (F59, green) antibodies at 30 hpf. B-D. Expression of flag-tagged TPR deletion mutant of Unc-45b (B) in fiber disrupts thick filament organization (C). D represents the merged image of B and C. Forty two myofibers from 36 embryos were analyzed. 75% of the myofiber showed the disorganized thick filaments. E-G. Deletion of the C-terminal UCS domain abolished the disruptive effect of Unc-45b overexpression. Myofiber expressing the UCS deletion mutant (E) exhibited normal thick filament organization (F). G represents the merged image of E and F. Twenty nine myofibers from 25 embryos were analyzed. None of the myofiber showed the disorganized thick filaments. Scale bars = 25 μm. |
|
Figure 1 (supplement) showed that overexpression of EGFP or Hsp90a1 had not effect on thick filament organization. A. DNA constructs expressing EGFP or myc-tagged Hsp90α1 in skeletal muscles of zebrafish embryos were directed using the smyd1 promoter. Myosin thick filament organization was analyzed by F59 staining. B-D. Single staining with anti-myosin (F59) antibody (red) shows that expression of GFP (B) has no effect on myosin thick filament organization (C, D). E-G. Double staining shows the expression of myc-tagged Hsp90a1 (E) in a single fiber and its lack of effect on myosin thick filament organization (F, G). G represents the merged image of E and F. Scale bars = 20 mm. |