- Title
-
Enrichment and differential targeting of complexins 3 and 4 in ribbon-containing sensory neurons during zebrafish development
- Authors
- Zanazzi, G., and Matthews, G.
- Source
- Full text @ Neural Dev.
|
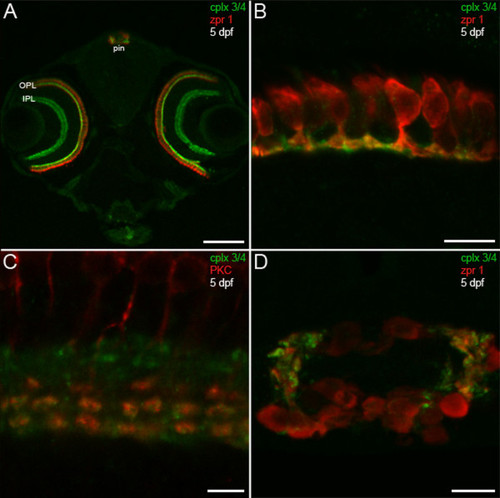
Predilection of complexin 3/4 for ribbon presynaptic terminals in the larval zebrafish visual system. (A) A confocal projection of a 5-dpf zebrafish transverse section stained with the pan-complexin 3/4 antibody (green) and the zpr 1/FRet 43 antibody (red), which labels double cone photoreceptors, reveals complexin 3/4 immunoreactivity throughout the retinal plexiform layers (OPL, IPL) and in the pineal organ (pin). Complexin 3/4 is absent from the retinal nuclear layers and the medial region of the pineal organ, which contains photoreceptor somata. (B) A high-magnification confocal projection of double cone photoreceptors (red) in the retinal OPL labeled with the complexin 3/4 antibody (green) shows that some of the complexin 3/4 immunoreactivity in the larval zebrafish OPL is found in double cone terminals. (C) Double-labeling with anti-protein kinase C (red) reveals overlap of complexin 3/4 (green) in ON bipolar cell terminals in the retinal IPL. (D) A confocal projection of a transverse section through the pineal organ reveals zpr 1/FRet 43-positive photoreceptors (red) and complexin 3/4 (green) in processes and terminals at the lateral border. Sections incubated with secondary antibodies alone exhibit background immunofluorescence in the retina and pineal (data not shown). Scale bars: 125 μm (A); 10 μm (B, D); 5 μm (C). EXPRESSION / LABELING:
|
|
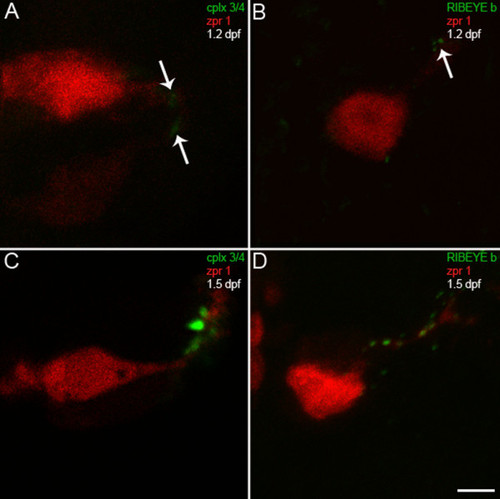
Complexin 3/4 concentrates in pineal photoreceptor terminals concomitant with RIBEYE b. (A) A high-magnification confocal projection of zpr 1/FRet 43-positive pineal double cone photoreceptors (red) double-stained for complexin 3/4 (green) at 1.2 dpf shows low levels of complexin in photoreceptor terminals (arrows). Ventral is toward the top and lateral is toward the right. (B) A section from a different embryo at 1.2 dpf double-stained with anti-zpr 1/FRet 43 (red) and anti-RIBEYE b (green) shows three small RIBEYE puncta in a pineal photoreceptor terminal (arrow). (C) By 1.5 dpf, complexin 3/4 (green) is highly expressed in neuropil at the lateral border of the pineal organ, in both zpr 1/FRet 43-positive (red) and zpr 1/FRet 43-negative photoreceptor axons and terminals. (D) Several RIBEYE b puncta (green) are present in pineal photoreceptor axons and terminals at 1.5 dpf. Scale bar = 5 μm. |
|
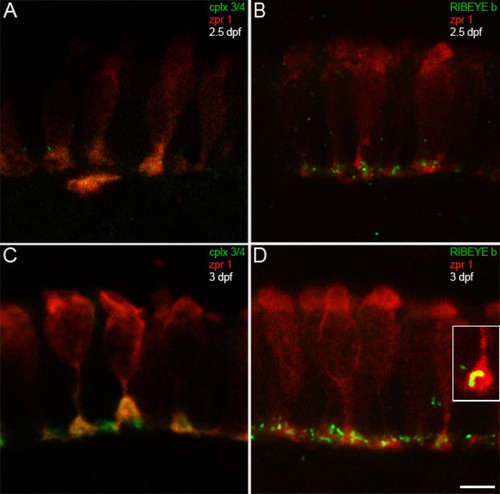
Complexin 3/4 concentrates in retinal photoreceptor terminals concomitant with RIBEYE b. (A) The zpr 1/FRet 43 monoclonal antibody (red) and the pan-complexin 3/4 polyclonal (green) were also used to localize these complexins in developing retinal photoreceptors. At 2.5 dpf, retinal photoreceptors are most prominent in the ventronasal patch, where complexin 3/4 immunoreactivity appears in some photoreceptor terminals. (B) RIBEYE b (green) has also started to cluster in photoreceptor terminals (red) in the outer plexiform layer at 2.5 dpf. (C) At 3 dpf, complexin 3/4 (red) is highly expressed in zpr 1/FRet 43-positive (red) and zpr 1/FRet 43-negative photoreceptor terminals in the ventronasal patch. (D) Pleiomorphic RIBEYE b (green) expression is found at 3 dpf in zpr 1/FRet 43-positive (red) and zpr 1/FRet 43-negative photoreceptor terminals in the ventronasal patch. The inset shows a zpr 1/FRet 43-positive terminal containing curvilinear RIBEYE b immunoreactivity that may correspond to a ribbon. Scale bar = 5 μm. EXPRESSION / LABELING:
|
|
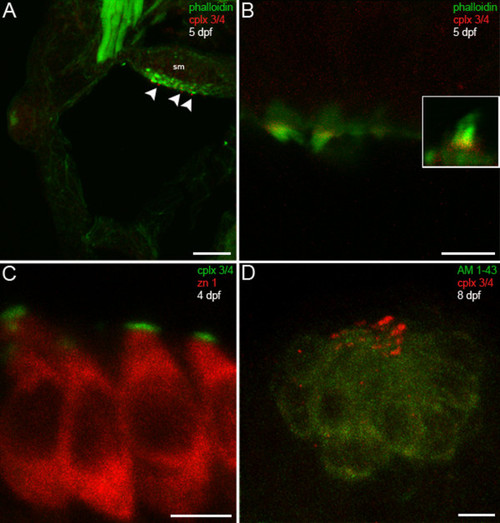
Hair cells in the larval zebrafish inner ear and lateral line exhibit complexin 3/4 immunoreactivity on their apical surfaces. (A) A confocal projection of a 5-dpf zebrafish transverse section through the otic vesicle reveals complexin 3/4 immunoreactivity (red) on the apical surfaces of inner ear hair cells in the saccular macula (sm; arrowheads) labeled with phalloidin (green). (B) A high-magnification en face view shows complexin 3/4 immunoreactivity (red) at the base of stereocilia labeled by phalloidin above the actin-rich cuticular plate. The inset contains an enlargement of a hair bundle. (C) Complexin 3/4 immunoreactivity (green) directly abuts zn 1 cytoplasmic immunoreactivity (red) on the apical surfaces of inner ear hair cells. (D) To examine neuromast hair cells more closely, larval zebrafish were incubated with AM1-43 (green), fixed, sectioned sagittally, and labeled with anti-complexin 3/4 (red). Complexin 3/4 immunoreactivity is also present on the apical surfaces of neuromast hair cells. Neuromast and inner ear sections incubated with secondary antibodies alone exhibit background immunofluorescence (data not shown). Scale bars: 25 μm (A); 5 μm (B-D). EXPRESSION / LABELING:
|
|
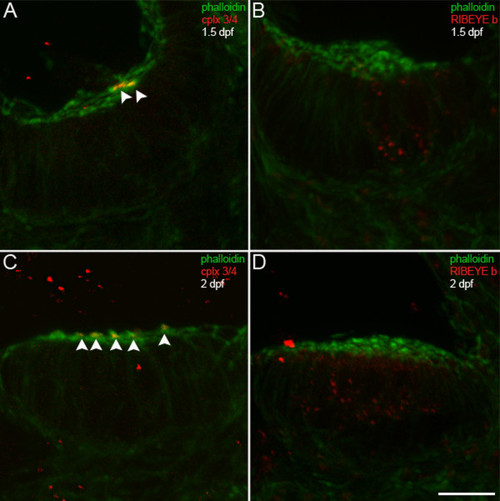
Complexin 3/4 does not co-localize with RIBEYE b in embryonic inner ear hair cells. (A) A high-magnification confocal projection through the posterior macula in the zebrafish inner ear at 1.5 dpf. Using phalloidin (green) as a marker of developing hair cell stereocilia, one can observe a small number of hair cells with stereocilia. Complexin 3/4 (red) is already apparent among these stereocilia (arrowheads). (B) Dual immunolabeling of RIBEYE b (red) and F-actin with phalloidin (green) in the anterior macula at 1.5 dpf. Note that RIBEYE b has clustered into large puncta on the basolateral membrane of hair cells with stereocilia. (C) At 2 dpf, many more hair cells with stereocilia (green) can be observed in the anterior macula. Complexin 3/4 immunoreactivity (red) can be observed among these stereocilia (arrowheads). (D) RIBEYE b (red) is upregulated in the cytoplasm of more hair cells in the anterior macula at 2 dpf. Scale bar = 10 μm. |
|
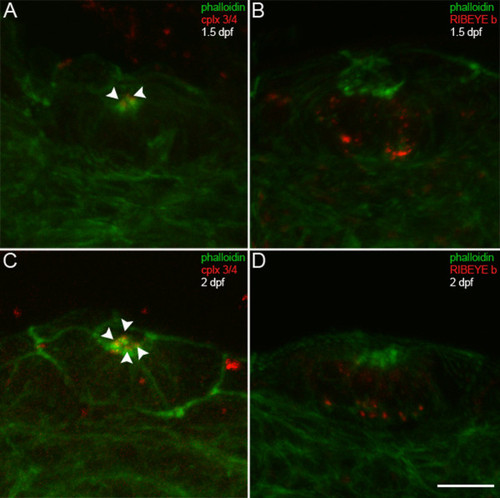
Complexin 3/4 does not co-localize with RIBEYE b in embryonic neuromast hair cells. (A) Embryonic zebrafish at 1.5 dpf were sectioned transversely. Phalloidin (green) and anti-complexin 3/4 (red) were used to probe otic neuromasts. Note that complexin 3/4 is weakly expressed among neuromast hair cell stereocilia at this time (arrowheads). (B) Large RIBEYE b puncta (red) can be observed at the base, and diffuse cytoplasmic immunoreactivity elsewhere, in a couple of neuromast hair cells at 1.5 dpf. (C) Greater numbers of hair cells in an otic neuromast have stereocilia (green) at 2 dpf. These hair cells also have apical complexin 3/4 immunoreactivity (red, arrowheads). (D) At 2 dpf, many hair cells in an otic neuromast have RIBEYE b immunoreactivity (red). Scale bar = 10 μm. |
|
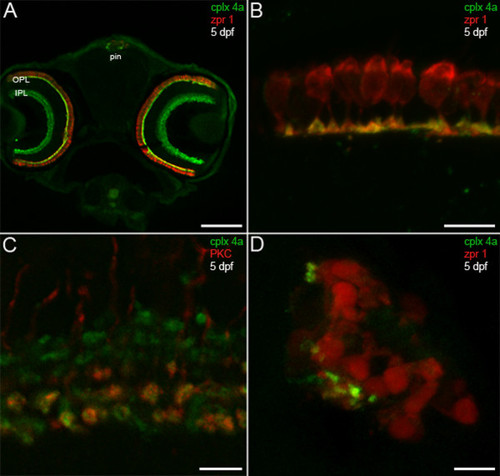
Complexin 4a marks visual system ribbon presynaptic terminals. (A) A confocal micrograph of a 5-dpf zebrafish transverse section stained with the anti-complexin 4a antibody (green) and anti-zpr 1/FRet 43 (red). Note the similarity between the complexin 4a expression pattern and that of complexin 3/4 (Figure 3A) at low magnification in the retinal plexiform layers (OPL, IPL) and pineal organ (pin). (B) At high magnification, complexin 4a appears to be restricted primarily to the terminals of double cone photoreceptors in the retinal outer plexiform layer. (C) A confocal planar projection of the retinal inner plexiform layer shows overlap of complexin 4a (green) in protein kinase C (PKC)-positive (red) ON bipolar cell terminals in the IPL. (D) A confocal planar projection of a 5-dpf zebrafish sagittal section through the pineal organ is shown. Zpr 1/FRet 43-positive photoreceptors (red) are oriented such that their outer segments are medial and their short axons and presynaptic terminals are lateral. Complexin 4a (green) specifically localizes to putative terminals. Sections incubated with secondary antibodies alone exhibit background immunofluorescence in the retina and pineal (data not shown). Scale bars: 125 μm (A); 10 μm (B, D); 5 μm (C). EXPRESSION / LABELING:
|
|
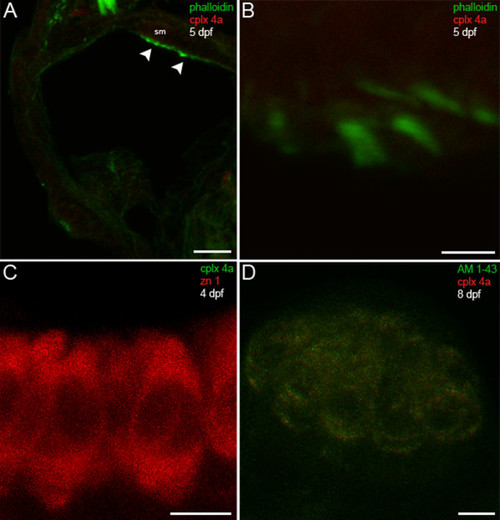
Complexin 4a is not expressed in hair cells of the larval zebrafish acousticolateral system. (A) A low-magnification confocal micrograph of a transverse section through the otic vesicle of a 5-dpf zebrafish shows saccular macula (sm) inner ear hair cell stereocilia stained with phalloidin (green) that lack complexin 4a (red, arrowheads). (B) A high-magnification en face view of inner ear hair cells labeled with phalloidin (green) confirms the absence of complexin 4a (red) among their stereocilia. (C) Inner ear hair cells incubated with anti-zn 1 (red) lack complexin 4a (green). (D) A high-magnification confocal planar projection through a cranial neuromast labeled with AM1-43 (green) indicates that complexin 4a is also absent from these hair cells. Sections incubated with only secondary antibodies exhibit very low levels of diffuse immunofluorescence throughout the hair cells (data not shown). Scale bars: 25 μm (A); 5 μm (B-D). EXPRESSION / LABELING:
|
|
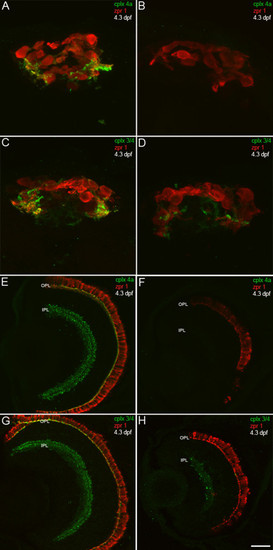
A complexin 4a morpholino antisense oligonucleotide knocks down complexin 4a protein expression in the larval zebrafish retina and pineal organ. (A-H) 4.3-dpf control (A, C, E, G) and complexin 4a morpholino-injected (B, D, F, H) larval zebrafish were sectioned transversely and stained with anti-zpr 1/FRet 43 (red) and either anti-complexin 4a (A, B, E, F) or anti-complexin 3/4 (C, D, G, H) (green). The complexin 4a translation-blocking morpholino dramatically decreases complexin 4a expression in the pineal organ (B) and in the retinal outer plexiform layer (OPL) and inner plexiform layer (IPL) (F). Some complexin 3/4 immunoreactivity remains in the pineal (D) and retina (H). Scale bars: 15 μm (A-D); 30 μm (E-H). EXPRESSION / LABELING:
|
|
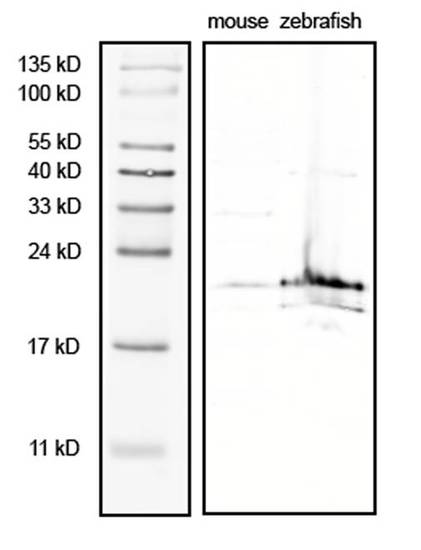
The complexin 3/4 antibody predominantly recognizes an approximately 20 kDa band on western blots. Adult mouse and zebrafish retinae were lysed in sample buffer containing protease inhibitors. Mouse extract (100 μg) and zebrafish extract (200 μg) were fractionated by SDS-PAGE (15% gel), blotted onto nitrocellulose, and probed with the complexin 3/4 polyclonal antibody (1:1,000). An approximately 20 kDa band is apparent in both mouse and zebrafish lysates. A minor band of approximately 17 kDa can also be observed in the zebrafish extract. EXPRESSION / LABELING:
|

Unillustrated author statements EXPRESSION / LABELING:
|