- Title
-
Arteries provide essential guidance cues for lymphatic endothelial cells in the zebrafish trunk
- Authors
- Bussmann, J., Bos, F.L., Urasaki, A., Kawakami, K., Duckers, H.J., and Schulte-Merker, S.
- Source
- Full text @ Development
|
Generation and visualization of flt1enh:RFP and SAGFF27C;UAS:GFP transgenic lines. (A-C) Arterial-restricted reporter expression in the flt1enh:RFP transgenic line at 5 dpf (A), compared with kdrl:GFP, which labels all blood endothelial cells (B) allows for discrimination between ISVs connected to the dorsal aorta or PCV. (C) Overlay of A,B. (D) Schematic representation of flt1enh:RFP expression. (E) Lymphatic reporter expression in the SAGFF27C;UAS:GFP transgenic line at 5 dpf, compared with an angiography (F) that labels perfused blood vessels (red in G). (G) Overlay of E,F. (H) Schematic representation of SAGFF27C;UAS:GFP expression. aISV, arterial ISV; DA, dorsal aorta; DLLV, dorsal longitudinal lymphatic vessel; ISLV, intersegmental lymphatic vessel; PCV, posterior cardinal vein; TD, thoracic duct; vISV, venous ISV. EXPRESSION / LABELING:
|
|
Arteriovenous and lymphatic patterning in the zebrafish trunk. (A) Schematic representation of arteries, veins and lymphatic vessels at 5 dpf, based on expression of flt1enh:RFP and SAGFF27C;UAS:GFP over 25-30 segments in 30 embryos (segment numbers vary in zebrafish embryos, with an average of 27.2 segments or 54.4 segmental positions per embryo). (B) Quantification of vessel type distribution per segment, indicating aISV in red bars, vISVs in blue bars and ISLVs in green bars. (C) Quantification of aISV/vISV identity in segments 5-25, n=1260 segmental positions scored. (D) Quantification of aISV/vISV identity of the caudal-most ISV/side, n=60 indicating a strong preference for aISV over vISV in the last ISV position. (E) Quantification of arterial and venous ISVs abutting an ISLV, n=430 intersegmental vessel pairs scored. (F) Overlaid confocal images of flt1enh:RFP+ aISV and SAGFF27C;UAS:GFP+ ISLV. aISV, arterial ISV; DA, dorsal aorta; DLLV, dorsal longitudinal lymphatic vessel; ISLV, intersegmental lymphatic vessel; TD, thoracic duct; vISV, venous ISV. EXPRESSION / LABELING:
|
|
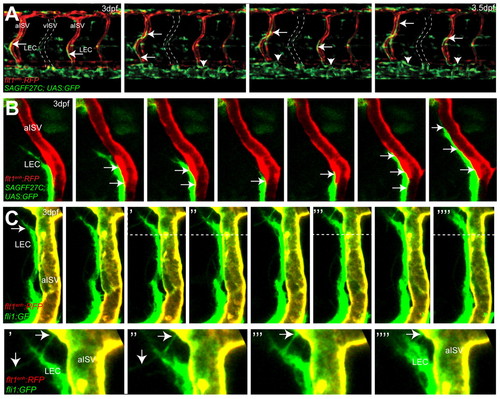
Migration of lymphatic precursors along intersegmental arteries. (A) Still images of flt1enh:RFP;SAGFF27C;UAS:GFP triple transgenic embryos reveal migration of SAGFF27C;UAS:GFP+ LECs (arrows) along flt1enh:RFP+ arteries and establishing the TD (arrowheads) in the trunk. The position of a vISV is indicated by broken lines. (B) Still images of migrating SAGFF27C;UAS:GFP+ LECs suggest direct contact (arrows) between the aISV and the LEC. (C) Still images of migrating fli1a:GFP+ LECs and filopodia formation (indicated by arrows) at the leading edge along the surface of a flt1enh:RFP+; fli1a:GFP+ artery at 3 dpf. Areas above the broken lines are shown at higher magnification in the bottom row. EXPRESSION / LABELING:
|
|
Intersegmental arteries are required for LEC migration. (A-C) flt1enh:RFP (A), SAGFF27C;UAS:GFP (B) and merged (C) unilateral confocal images of control embryos at 4.5 dpf showing lymphatic patterning along aISVs in normal blood vasculature. Arrows indicate ISLVs. (D-F) flt1enh:RFP (D), SAGFF27C;UAS:GFP (E) and merged (F) unilateral confocal images of kdrl morpholino injected embryos at 4.5 dpf showing LEC migration along arteries that cross-connect abnormally at the horizontal myoseptum, and that do not extend into the dorsal half (indicated by yellow asterisk) of the respective somite. Arrows indicate LECs along the surface of an abnormal aISV. Vertical red bars indicate the DA. (G) Model of lymphatic patterning within the zebrafish trunk. Secondary sprouts from the PCV that do not connect to primary intersegmental vessels migrate to the horizontal myoseptum region where they constitute a pool of PLs. PLs will migrate ventrally or dorsally to form the thoracic duct and ISLVs, respectively, and use arteries as their substrate. |
|
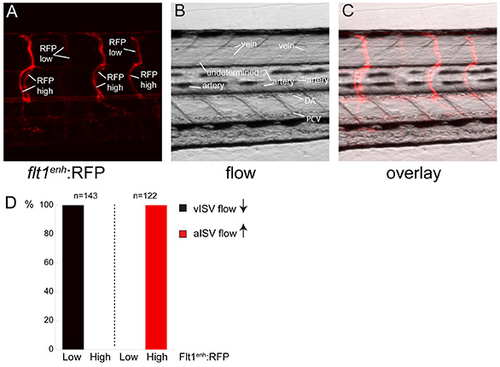
Comparison between flt1enh:RFP expression and direction of blood flow in the trunk intersegmental network. (A) flt1enh:RFP expression at 5 dpf reveals two types of ISVs with either high or low RFP expression. (B) Still frame of direction of flow in a 5 dpf flt1enh:RFP embryo revealing arteries (up from DA) and veins (down to PCV) (C) Overlay of flt1enh:RFP and flow reveals that arteries express flt1enh:RFP at high and veins express flt1enh:RFP at low levels. (D) Quantification of flow direction and expression of flt1enh:RFP. n=143 aISV and n=122 vISV. |
|
Arteries are required for lymphatic development. (A-C) 60 hpf sibling and mutant kdrlhu5088 in fli1a:GFP; flt1enh:RFP double transgenic embryos reveal that PL formation is normal, whereas the primary sprouts can be affected differently (indicated by the yellow asterisk) in mutants. (D) Kdrlhu5088 failed to complement kdrlt21588 (Habeck et al., 2002) and sequencing revealed an A-to-T transversion, changing a lysine at amino acid position 587 into a premature stop (K587X) resulting in reduced kdrl expression. (E,F) Lymphatic vasculature establishment in 4.5 dpf sibling and mutant kdrlhu5088 fli1:GFP; flt1enh:RFP double transgenic embryos with normal or with reduced primary sprouts at 4.5 dpf. Mutants have reduced primary sprouting (indicated by blue asterisks) and lack dorsal migration of LECs (indicated by yellow asterisks), whereas ventral migration is still present (G). Quantification of dorsal and ventral migration along arteries. Mutants with thoracic duct (n=5, total of 23 arteries) without dorsal migration, whereas the ventral migration along these arteries is mostly retained. (H,I) kdrlhu5088 escapers in fli1:GFP, flt1enh:RFP double transgenic embryos at 4.5 dpf. LECs (arrows) migrate along escaping arteries that did succeed in growing dorsally. |