- Title
-
Sec24D-dependent transport of extracellular matrix proteins is required for zebrafish skeletal morphogenesis
- Authors
- Sarmah, S., Barrallo-Gimeno, A., Melville, D.B., Topczewski, J., Solnica-Krezel, L., and Knapik, E.W.
- Source
- Full text @ PLoS One
|
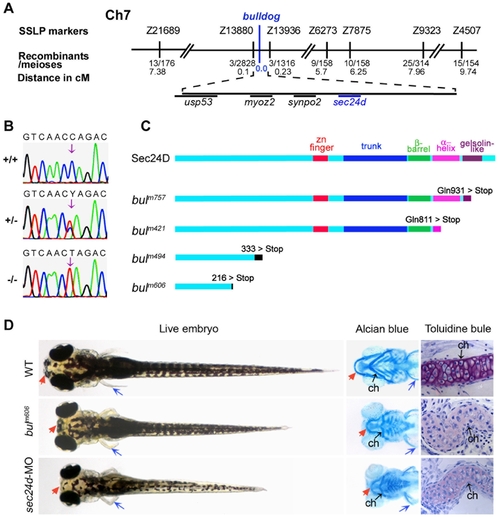
The bulldog mutations disrupt the sec24d gene and affect craniofacial development. (A) The bulldog mutations map to chromosome 7 between markers Z13880 and Z13936. The number of recombinants and the corresponding distances in centiMorgans (cM) are indicated below. The genes in the critical region are listed. (B) Electropherograms of wild-type +/+, heterozygous +/- and bulm421 -/- genomic DNA shows the C→T transition (arrows) that results in an amber stop codon (TAG) in place of glutamine at position 811 (Q811X). (C) Schematic diagram of the Sec24D primary protein structure of wild-type and bulldog mutants. (D) bulm606 and sec24d morphant embryos (3.5 ng sec24d-MO) have reduced head size (red arrows), shorter body length and pectoral fins (blue arrows). Alcian blue staining of the head skeleton in the right panels shows short Meckel′s (red arrows), deformed ceratohyals (black arrows) and kinked pectoral fins (blue arrows) in bulm606 and sec24d morphant embryos compared to wild types. Toluidine blue stained coronal sections of craniofacial cartilage in bulm606 and sec24d morphant embryos demonstrate very low amount of ECM material (purple staining) and abnormal shape and packing density of chondrocytes as compared to wild types, all at 4 dpf. Abbreviation: ch, ceratohyal. PHENOTYPE:
|
|
sec24d mRNA expression during embryogenesis and molecular analysis of the bulldog mutation. (A) RT-PCR assay shows maternally deposited sec24d transcripts at 1 and 2 hpf and increasing levels thereafter. β-actin served as control. (B–H) Expression patterns of sec24d during zebrafish development using whole mount in situ hybridization staining of wild-type embryos. The cross section through the palatoquadrate (pq, arrowheads) at 3 dpf (I) shows robust riboprobe staining (h: hours, d: days). (J–O) Expression patterns of col2α1 (J,K) and sox9a (L,M) at 3 dpf. The expression is slightly upregulated in the posterior pharyngeal cartilages of bulm606 embryos. The Collagen-specific chaperone hsp47 (N,O) transcript is elevated in craniofacial skeleton (arrowheads), fins and the notochord. Arrowheads point to the jaw region in panels (G) through (O). (P) Relative sec24d sec24c, bip and sil1 mRNA levels were assessed by quantitative real-time PCR and adjusted against β-actin using total RNA samples from 4 dpf embryos. In bulm494, mRNA levels for bip and sil1 are induced 5-fold, whereas sec24d mRNA levels are reduced 0.7-fold. *, p<0.02; **, p<0.005. Abbreviation: b, brain, o, optic nerve, ot, otic capsule, r, retina. Anterior (D–O) is to the left. PHENOTYPE:
|
|
Sec24D-deficient cartilage contains sparse ECM and chondrocytes with distended ER. (A–H) Electron micrographs show mature wild-type chondrocytes (cells 1–3 in A) are separated by ECM and contain dense ER membranes (A, E, arrows). In bulm494 mutants (B,F,H), cartilage matrix is sparse and large amounts of electron-dense material accumulate in the rough ER (asterisks, cells marked 1–6 in B). Wild-type chondrocytes (C) are devoid of cell-cell contacts, whereas the majority of bulm494 mutant cells (D) contain adherens junctions (arrowheads). bulm494 chondrocytes (F) contain both normal ER cisternae (arrows) and swollen, ribosome studded, ER (asterisks). sec24d-deficient cells (H) contain smaller and disorganized Golgi complexes (blue arrows) compared to wild-types (G). (I,J) Single-pass confocal images of C6-NBD ceramide stained chondrocytes of wild-type (I) and sec24d/bulm606 mutant (J) embryos at 4 dpf. (K,L) Adherens junctions are marked by β-catenin (red) in bulldog Meckel′s cartilage (arrowheads), but are mostly absent in wild types (K). (M,N) Phalloidin (red) shows regular cortical distribution of polymerized actin in wild types (M), but uneven cellular distribution in bulm606 cartilage (arrowheads) (N). Nuclei are stained with TOPRO-3 (blue). Abbreviations: m, mitochondria; n, nucleus; ECM, extracellular matrix. Scale bars: 2 μM (A,B,E); 500 nm (F–H); 5 μM (I–N). PHENOTYPE:
|
|
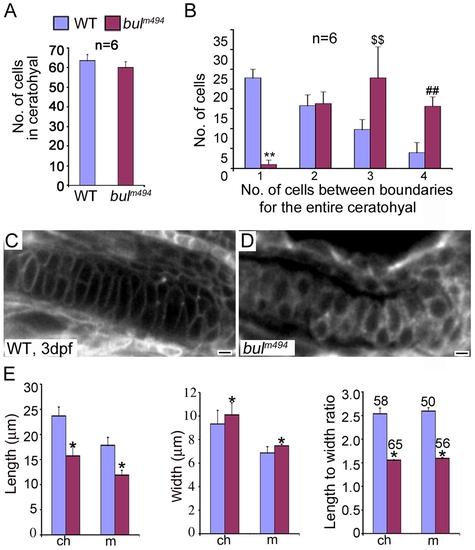
Analysis of chondrocyte shape and numbers in bulldog mutants. (A) The number of cells in the ceratohyals at 5 dpf is not significantly different between wild-type and bulldog embryos (counted in single optical plane of Alcian blue stained preparations, six different animals each). (B) In contrast, the number of cells spanning the entire width of the ceratohyal at 5 dpf is notably higher in bulldog. (C,D) Single-pass confocal images of the Meckel′s cartilage in live embryos marked with membrane tethered GFP tracer. Bulldog mutants (D) show multiple stacked chondrocytes as compared to a single spanning cell in wild-types (C). (E) The average chondrocyte width is comparable between wild-type and bulldog, whereas the length and the length-to-width ratio are significantly lower in the mutants. Cellular dimensions were counted in Meckel′s (m) and ceratohyal (ch) cartilages in three different live embryos at 3 dpf. The number of cells used for measurements is indicated in the right graph (E). * denotes p<0.0001; **, p<0.0001; $$, p<0.003; ##, p<0.0001. PHENOTYPE:
|
|
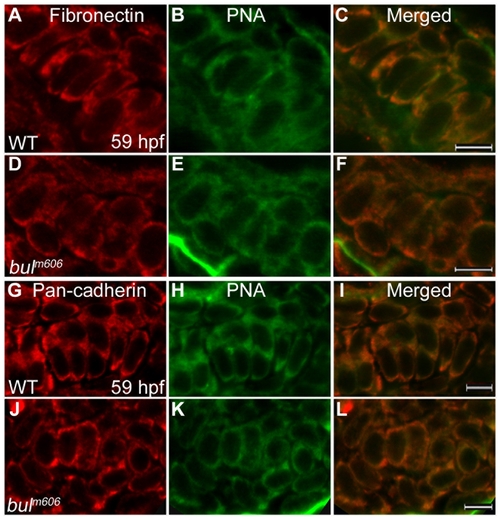
Analysis of mesenchymal condensations in cartilage primordia of bulldog mutants. (A–F) Mesenchymal condensations, stained with Peanut Agglutinin (PNA, green) show similar distribution of Fibronectin (red) in wild-type (A–C) and bulldog hyosymplectic cartilages (D–F). (G–L) Cadherin expression in chondrocytes (marked by a Pan-cadherin antibody in red) is indistinguishable between wild-type and bulldog ceratohyals as shown by PNA staining. The right panels represent merged images of the left and middle panels. Scale bars are 5 μM. PHENOTYPE:
|
|
Sec24D-deficient chondrocytes do not transport ECM proteins. (A–F) Collagen2α1 (red) and Wheat Germ Agglutinin (WGA, green) signals are concentrated at juxtanuclear compartments and in the extracellular space of wild-type chondrocytes (A–C). In bulldog, the signals are primarily localized in cytoplasmic vesicular compartments, although weak WGA signal is also detectable at the plasma membrane and the extracellular space (D–F). (G–L) Single pass confocal images of wild-type and bulm606 Meckel′s cartilages labeled with the β1-integrin (red) recognizing antibody and counterstained with WGA (green). Merged channels show co-localization of the two labels in the cell boundaries in both wild-type and bulldog chondrocytes. The right panels represent merged images of the left and middle panels. Scale bars are 5 μM. |
|
Sec24C expression during zebrafish development. (A,B) sec24c is ubiquitously expressed at cleavage (8-cell, A) and gastrulation (shield, B) stages. (C–F) Sec24c expression at consecutive embryonic days is concentrated in the head and gut regions. Expression in the head overlaps with developing craniofacial structures. |
|
Knockdown of sec24c, and combined loss of sec24d and sec24c phenotypes. Analysis of wild types (A, E, I), sec24c-UTR-MO (B,F,J), bulldog (C, G, K) and double sec24c-UTR-MO+ sec24d-UTR-MO (D, H, L) injected embryos. (A–D) Images of live wild types (A), sec24c-UTR-MO, (2.0 ng injected, B), bulldog (C) and sec24c-UTR-MO+ sec24d-UTR-MO (2.0 ng each, D). The sec24c morphants have reduced body length (blue arrows), but the head appears normal. Double morphants for sec24d+sec24c are significantly shorter than bulldog larvae, almost completely lack fin-folds and have reduced jaw region (red arrow) and pronounced heart edema (D). (E–H) Alcian blue preparations of the head skeleton. The Alcian blue staining confirms normal craniofacial cartilages in sec24c morphants. (I–L) Histological sections counter-stained with toluidine blue at 4 dpf. Insets indicate the plane of the sections. (I,J) Histological, sagittal plastic sections stained with toluidine blue show normal jaw opening and surrounding cartilages in wild types (I) and sec24c morphants (J). Purple staining patterns for glycosaminoglycans of the ECM are also comparable. In contrast, the pharyngeal skeleton of sec24d+sec24c double mutants fails to stain with Alcian blue (H). Histological sections reveal patterned arches that are largely devoid of metachromatically stained cells (L). Abbreviations: cb, ceratobranchial; ch, ceratohyal; ep, ethmoid plate, m, Meckel′s cartilage. PHENOTYPE:
|
|
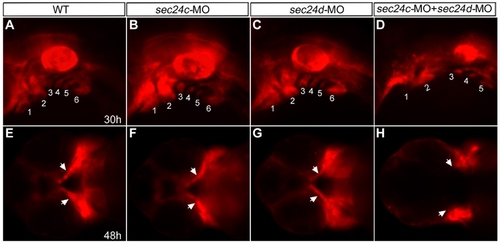
Migration of craniofacial primordia in double Sec24C and Sec24D morants. (A–H) Images of transgenic sox10:mRFP embryos at 30 hpf (lateral view A–D) and 48 hpf (ventral view E–H) after injection with sec24c-UTR-MO, (2.0 ng), sec24d-UTR-MO (3.5 ng), and sec24c-UTR-MO+ sec24d-UTR-MO. Neural crest streams are numbered (1, 2, 3, 4, 5, 6 in A–D). Arrows point to migrating craniofacial primordia (E–H). |
|
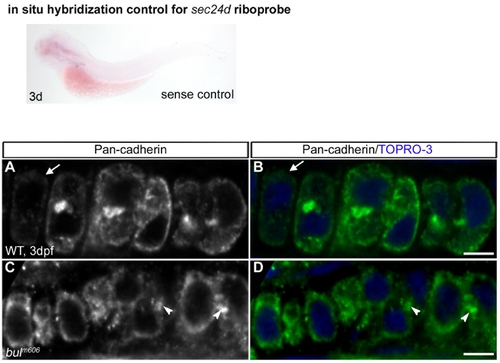
Sense control for the expression of sec24d in 3 dpf embryo by in situ hybridization shows no staining. Adherence Junctions Persist in bulldog Mutant Chondrocytes. (A–D) Pan-cadherin antibody predominantly labels N-cadherin in 3 dpf chondrocytes (green). Cadherin staining disappear as wild-type chondrocytes begin to lose cell-cell contacts (arrow in A,B). In contrast, bulldog chondrocytes (C,D) express uniform levels of Cadherin in all cells that is concentrated at cell-cell contact points (arrowheads). Nuclei are stained blue with TOPRO-3. Scale bars are 5 μM. |
|
Cartilage Matrix Proteins are not Trafficked in bulldog Chondrocytes. Loss of Sec24D blocks ECM protein secretion in mature chondrocytes. (A–F) Overview of the lower jaw at 59 hpf labeled for Col2α1 in wild-type and bulldog embryos. The head is compact in wild types and laid out in more cuboidal shape, thus appearing smaller in the images (A,C,E), as compared to bulldog jaws that are wider and more flat along the Z axis (B,D,F). Type II collagen (A,B) is not trafficked in bulldog chondrocytes (B) at 59 hpf. Boxed areas are enlarged as insets. Wheat Germ Agglutinin (WGA)-labeled glycoproteins (C,D) are primarily localized to the extracellular space in the epithelial membranes of wild-type and bulldog embryos, but are weakly expressed in bulldog chondrocytes. The corresponding superimposed images are shown in E, F. Nuclei are stained blue with TOPRO-3. The insets in E,F are higher magnifications showing that the two labels mark distinct compartments, with WGA likely staining the Golgi complexes and the Col2α1 antibody the ER. (G–L) Co-staining of Col2α1 and WGA at 4 dpf in the first pharyngeal arch cartilages shows colocalization in the extracellular space in wild types (G–K), and accumulation in large vesicular structures in bullog mutants (H–L). (M–R) Matrilin, an extracellular matrix protein, is localized in the extracellular space of the basihyal cartilage and in tissues surrounding individual cartilage elements at 5 dpf in wild types (M), but it accumulates within cells in bulldog embryos (N). Boxed areas are enlarged. Counterstaining with WGA is shown in O and P. The large, round stained dots in wild-type and bulldog samples are the mucin producing cells of the digestive system present in both whole mount preparations (arrowheads). Abbreviations: m: Meckel′s cartilage; bh - basihyal cartilages; ch - ceratohyal cartilages. Scale bars are 20 μM. Methods: Immunofluorescence experiments were performed with 1:250 diluted primary antibodies against Matrilin (generous gift of Dr. E. Kremmer). Anti-rat Alexa Fluor 555 (Invitrogen) was applied as fluorescently-conjugated secondary antibody (1:400). |
|
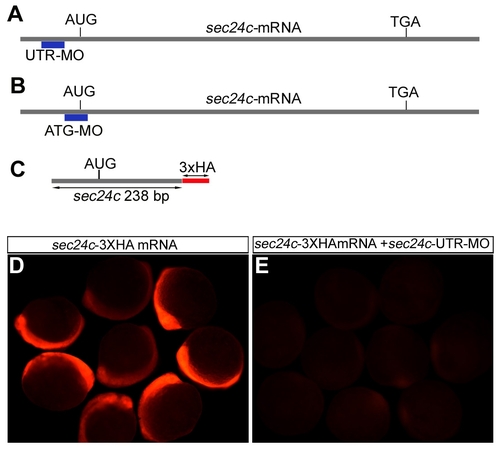
Sec24c knockdown strategy. (A,B) Schematic representation of the knockdown strategy for sec24c and the location of the translation blocking morpholinos: UTR-MO (A), ATG-MO (B). (C) Schematic drawing of the construct containing 238 bp of sec24c mRNA fused in frame to three HA tags in the pCS2+ vector that was used to test the effectiveness of translation inhibition by the morpholinos. (D,E) Injection of 100 pg of the HA tagged construct into 1-cell stage embryos shows expression as visualized by HA tag recognizing antibody in red (D). Injection of 100 pg of the HA tagged construct in combination with 2.0 ng of MO effectively blocked translation of the reporter construct (E). |