- Title
-
no tail integrates two modes of mesoderm induction
- Authors
- Harvey, S.A., Tümpel, S., Dubrulle, J., Schier, A.F., and Smith, J.C.
- Source
- Full text @ Development
|
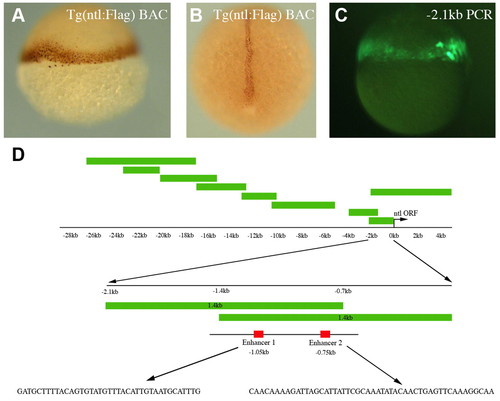
Mapping the ntl regulatory elements. (A,B) Anti-Flag immunostaining of ntl:Flag BAC transgenic embryos shows expression in the margin at 6 hpf (A) and in the notochord at 11 hpf (B). (C) An embryo injected with a -2.1 kb ntl promoter PCR fragment activates GFP expression in the margin at 6 hpf. (D) An illustration of the PCR fragments generated to map the ntl regulatory elements. Two overlapping 1.4 kb fragments displayed the same enhancer properties as the 2.1 kb fragment. Within the overlapping 700 bp region (-0.7 kb to -1.4 kb) we focused on enhancer 1 (E1, 40 bp) and enhancer 2 (E2, 50 bp). The sequences of E1 and E2 are shown. |
|
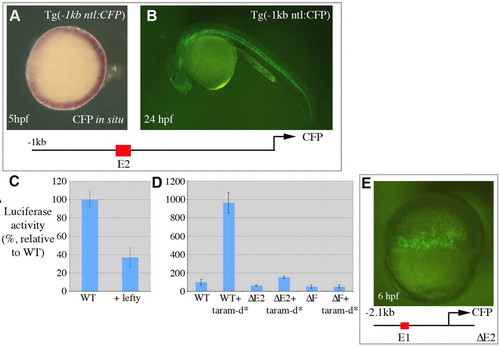
Nodal-dependent activation of ntl expression. (A,B) A transgenic line with -1 kb of the ntl promoter upstream of CFP. (A) An animal pole view shows transgene expression in the developing margin. (B) The transgene is also expressed in the developing notochord as shown at 24 hpf. (C,D) Luciferase assays performed at 6 hpf with the -2.1 kb ntl promoter fragment. (D) Wild type, -2.1 kb ntl promoter. ΔE2, -2.1 kb ntl promoter, where E2 (50 bp) was removed. ΔF, -2.1 kb ntl promoter with the putative fast-binding site (AAATATACA) removed from E2. (E) An embryo injected with a plasmid containing the -2.1 kb promoter fragment where E2 (50 bp) has been deleted (-2.1 kb ΔE2) upstream of CFP. Expression of CFP was observed in the developing margin at 6 hpf, but not in the notochord at 24 hpf. |
|
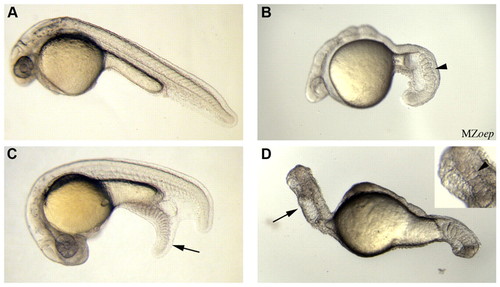
Tail formation in the absence of nodal signalling. (A) A wild-type embryo. (B) An MZoep embryo, with an arrowhead highlighting somites within the tail region. (C,D) Embryos previously injected at the 128-cell stage with zebrafish bmp4 and wnt8 (50 pg of each). (C) Injection of bmp4 and wnt8 into wild-type embryos produced an ectopic tail (arrow) containing somites. Ectopic tails formed in 22% of injected embryos (n=117). Injection of Xenopus laevis BMP4 and Wnt8 induced ectopic tails in 18% of injected embryos (data not shown, n=114). (D) Injection of bmp4 and wnt8 into MZoep embryos led to the formation of an ecoptic tail (arrow) in 29% of injected embryos (n=29). A magnified image of the ectopic tail (inset), which was identified by co-injection of a marker (not shown), highlights somites within the tail (arrowhead). |
|
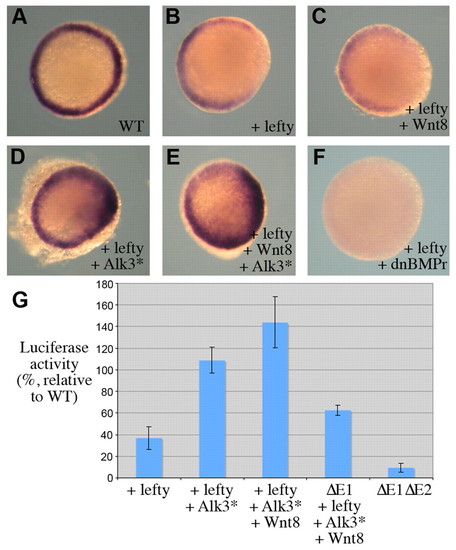
Nodal-independent regulation of ntl. (A-F) Animal pole views of ntl RNA in situ hybridizations at 6 hpf. (A) A wild-type embryo. Embryos injected with lefty (B) (100% with displayed expression, n=61), lefty + wnt8 (C) (100% with displayed expression, n=42), lefty + Alk3* (D) (85% increased expression compared with B, n=40), lefty + wnt8 + Alk3* (E) (87% increased expression compared with B, n=79), lefty + dnBMPr (F) (80% no expression, n=40). (G) Luciferase assays performed at 6 hpf with the -2.1 kb ntl promoter fragment in wild-type embryos and embryos injected with RNA encoding Wnt8 and/or Alk3*. ΔE1 = -2.1 kb ntl promoter where E1 (40 bp) was present. ΔE1ΔE2 = -2.1 kb ntl promoter where E1 and E2 were removed. |
|
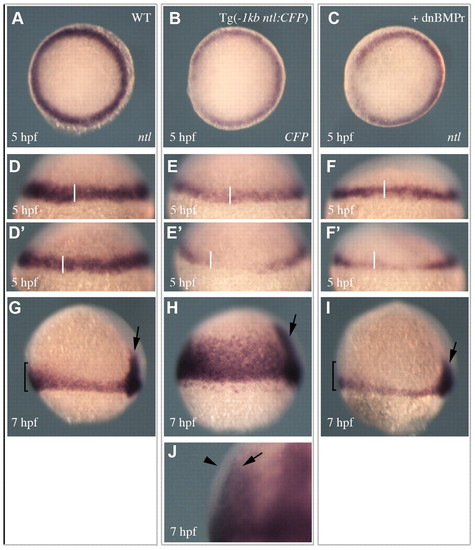
Spatial and temporal regulation of ntl expression. (A,D,D′,G) ntl expression in wild-type embryos. (B,E,E′,H,J) Expression of the -1 kb ntl promoter transgene. (C,F,F′,I) The expression of ntl in embryos injected with a dnBMPr. (A-C) Animal pole views of expression at 5 hpf. (D-F′) Views of expression on opposing sides of individual embryos at 5 hpf (transgene expression 84%, n=51 and dnBMPRr injected 69%, n=46). (G-I) Lateral views of expression at 7 hpf. Arrows indicate expression in the developing notochord. Brackets in G and I highlight the enriched expression in the ventral margin of wild-type embryos, which is not observed in embryos injected with the dnBMPr (92%, n=37). (J) A magnified lateral view of a 7 hpf embryo shows the transgene is expressed in the underlying endodermal cells (arrow) and not the outer mesodermal cells (arrowhead) (100%, n=52). |
|
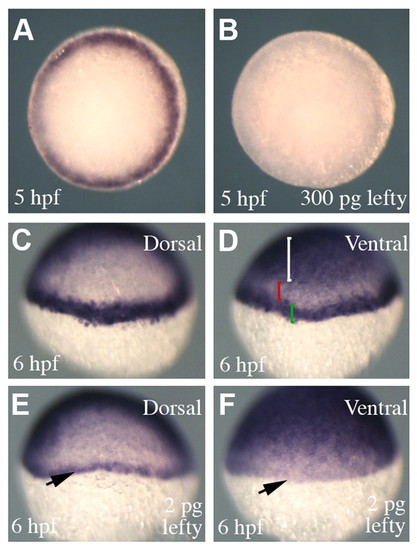
Attenuation of nodal signalling in Tg(-1 kb ntl:CFP) embryos. (A,B) Animal pole views showing transgene expression in Tg(-1 kb ntl:CFP) embryos. Injection of high concentrations of lefty (300 pg) completely abolished transgene expression (B) (100%, n=68). (C,D) A transgenic embryo processed to show transgene and gata2 expression, which marks the ventral ectoderm. (D) The white bracket highlights gata2 expression in the ventral ectoderm and the green bracket shows transgene expression in the margin. The red bracket shows a region that separates the expression domains of gata2 and the transgene. (E,F) A transgenic embryo injected with a low concentration of lefty (2 pg). (E) On the dorsal side of the embryo the transgene is expressed in fewer cell tiers compared with uninjected embryos (C). However, within the same embryo, transgene expression is abolished in the ventral margin (arrow) (expression pattern observed in 43%; in 50% transgene expression was completely abolished, n=60). |
|
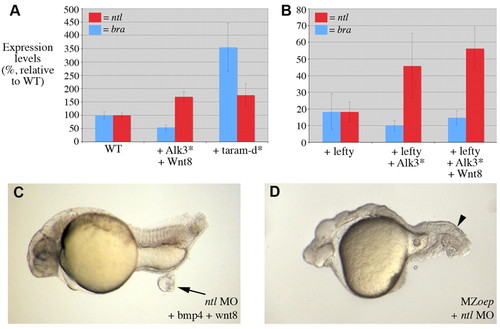
Nodal-independent regulation of ntl is essential for tail formation. (A,B) Quantitative RT-PCR of ntl (red) and bra (blue) expression at 6 hpf. Absolute levels of wild-type ntl expression were 1.44 times greater than that of bra. (A) Embryos injected with Alk3* and wnt8 displayed an increase in ntl, but not bra expression. Injection of taram-d* produced an increase in both ntl and bra expression. (B) Injection of embryos with lefty and then Alk3* and wnt8 demonstrated that in the absence of nodal signalling BMP and Wnt signalling can activate the expression of ntl but not bra. (C) An embryo injected with an ntl morpholino and then further injected in the animal pole with bmp4 and wnt8 leads to a small ectopic mass (arrow) in 29% of embryos (n=77) (compare with Fig. 3C). (D) An MZoep embryo injected at the one-cell stage with an ntl MO. Loss of ntl function abolished the formation of somites within the tail region of MZoep embryos (arrowhead, compare with Fig. 3B). |
|
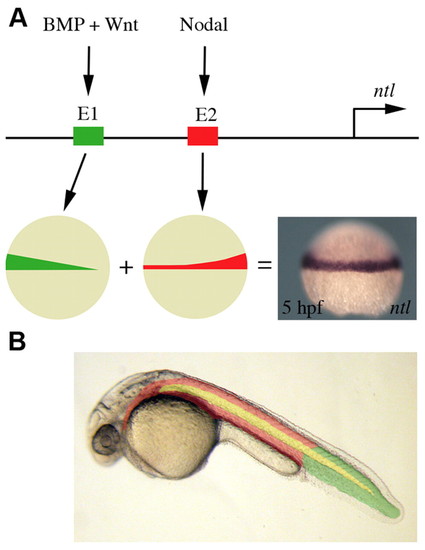
A model of ntl regulation. (A) The ntl promoter contains two different regulatory elements that activate ntl expression in the developing margin. BMP and Wnt signalling acts through one element (E1, shown in green) to activate ntl expression in the ventral margin. Nodal signalling induces ntl expression via a separate regulatory element (E2, shown in red), resulting in an enrichment of expression in the dorsal margin. (B) BMP and Wnt induction of ntl is required for tail mesoderm formation (shown in green). Anterior mesoderm (shown in red) results from the induction of ntl and ntl expression by nodal signalling. Notochord formation (shown in yellow) is dependent on regulatory elements that also lie within E2 of the ntl promoter. |