- Title
-
Broad-Minded Links Cell Cycle-Related Kinase to Cilia Assembly and Hedgehog Signal Transduction
- Authors
- Ko, H.W., Norman, R.X., Tran, J., Fuller, K.P., Fukuda, M., and Eggenschwiler, J.T.
- Source
- Full text @ Dev. Cell
|
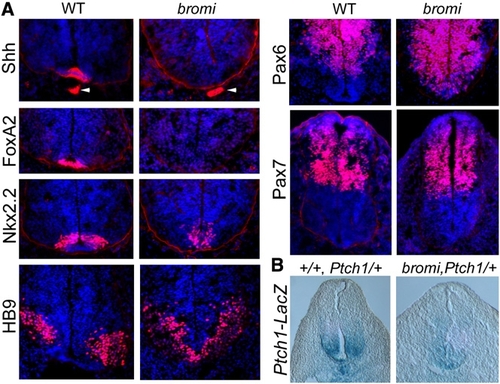
Neural Patterning in bromi Mutants |
|
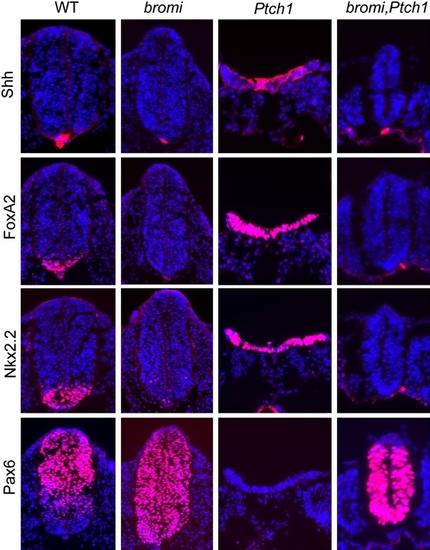
bromi Is Epistatic to Ptch1 |
|
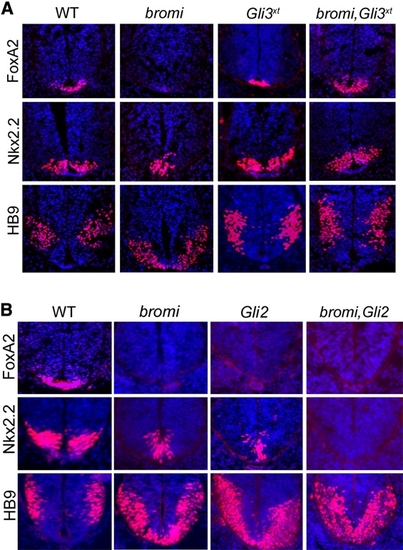
Patterning in bromi,Gli3xt and bromi,Gli2 Double Mutants |
|
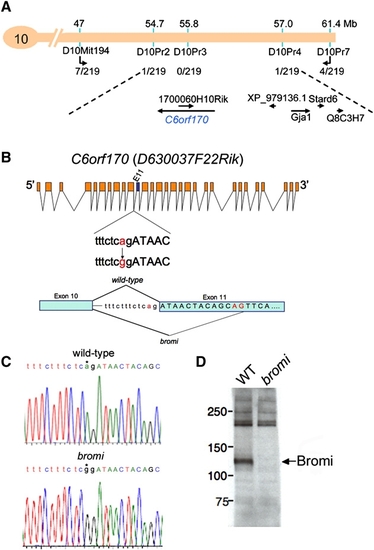
Positional Cloning of bromi |
|
Cilia Defects in bromi Mutants |
|
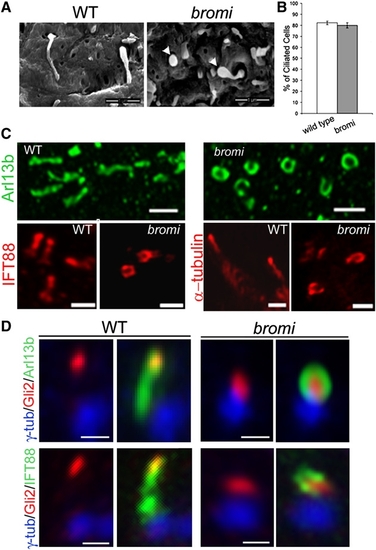
bromi Regulates the Association Between Ciliary Membranes and Axonemes EXPRESSION / LABELING:
PHENOTYPE:
|
|
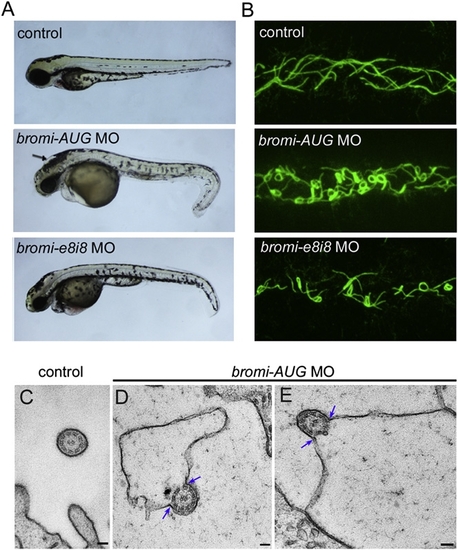
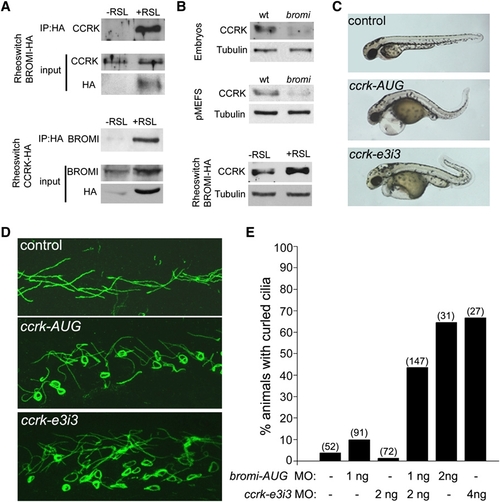
CCRK Interacts with Bromi and Acts in Vertebrate Ciliogenesis EXPRESSION / LABELING:
PHENOTYPE:
|
Reprinted from Developmental Cell, 18(2), Ko, H.W., Norman, R.X., Tran, J., Fuller, K.P., Fukuda, M., and Eggenschwiler, J.T., Broad-Minded Links Cell Cycle-Related Kinase to Cilia Assembly and Hedgehog Signal Transduction, 237-247, Copyright (2010) with permission from Elsevier. Full text @ Dev. Cell