- Title
-
The zebrafish mutant bumper shows a hyperproliferation of lens epithelial cells and fibre cell degeneration leading to functional blindness
- Authors
- Schonthaler, H.B., Franz-Odendaal, T.A., Hodel, C., Gehring, I., Geisler, R., Schwarz, H., Neuhauss, S.C., and Dahm, R.
- Source
- Full text @ Mech. Dev.
|
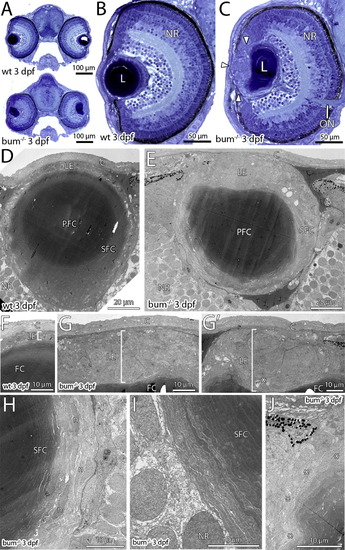
The lens in 3 dpf bum-/- zebrafish shows the onset of a hyperproliferation of the anterior epithelium. (A–C) Toluidine blue-stained transversal semi-thin sections through the central part of the eyes of 3 dpf sibling (wild-type, wt; top picture in A and B) and bum-/- mutant zebrafish larvae (bottom picture in A and C). (A) Cross-sections of entire heads, (B and C) higher magnifications of individual eyes. Note the onset of a hyperproliferation of the anterior lens epithelium in the mutant (C; white arrowheads). The overall morphology of the eye however, including the layers of the neural retina (NR), is normal in bum-/- mutant larvae (compare B and C). (D–J) Thin-section electron microscopy images of transversal sections through the central part of the eyes of 3 dpf sibling (D, F) and bum-/- (E, G–J) zebrafish larvae. (D, E) Overviews showing the morphology of the entire lens. (F, G) Higher magnifications of the anterior lens epithelia in sibling (F) and bum-/- (G, G′) zebrafish larvae. Note that the epithelium (brackets) forms a monolayer in the wild-type larvae, but is expanded to several cell layers in the mutants. Occasionally, apoptotic cells can be observed in the mutant lens epithelium (asterisk in G′). (H, I) Higher magnifications of the lens’ bow region in bum-/- mutant zebrafish larvae showing the differentiating secondary lens fibre cells, which at this stage do not yet show the massive degenerative changes observed at later developmental stages (see [Fig. 2] and [Fig. 4]). (J) Slight deformation of the outer cortex of a bum-/- mutant lens anterior of the lens’ equator. Asterisks denote superficial secondary lens fibre cells that show the first signs of cellular swelling. Abbreviations: C, cornea; PFC, primary lens fibre cells; L, lens; LE, lens epithelium; n, secondary lens fibre cell nuclei; NR, neural retina; ON, optic nerve; P, pigment granules of the iris; PFC, primary lens fibres; SFC, secondary lens fibre cells. PHENOTYPE:
|
|
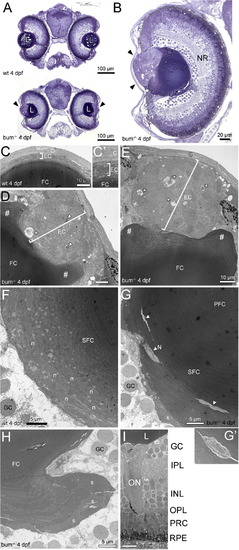
The lens in 4 dpf bum-/- zebrafish shows a massive hyperproliferation of the anterior epithelium and the onset of a maldifferentiation of secondary fibre cells. (A, B) Toluidine blue-stained transversal semi-thin sections through the central part of the eyes of 4 dpf sibling (wild-type, wt; top picture in A) and bum-/- zebrafish larvae (bottom picture in A and B). (A) Cross-sections of entire heads, (B) A higher magnification of an individual eye. Note the hyperproliferation of the anterior lens epithelium in the mutant (black arrowheads), which does not affect the differentiation of the primary and early developing secondary fibre cells (collectively termed “FC” here as they cannot be differentiated at the magnification shown in B). The overall morphology of the head (A) and eye (B), including the development of the layers of the neural retina (NR), are normal in bum-/- mutant larvae. (C–I) Thin-section electron microscopy images of transversal sections through the central part of the eyes of 4 dpf bum-/- zebrafish larvae. (C–E) Anterior part of the eyes of 4 dpf sibling (C; higher magnification C′) and bum-/- mutant (D, E) zebrafish larvae. Note that while wild-type larvae consistently have a monolayered lens epithelium (brackets in C, C′), the mutants display a massively hyperproliferating lens epithelium (brackets in D, E). Moreover, the mutant lens epithelium frequently comprises pyknotic nuclei (arrowheads in D) and empty spaces containing cell debris (asterisks in D and E). The # signs indicate the massive deformation of the fibre mass near the anterior lens pole. (F, G) Bow regions posterior of lens equator encompassing the early differentiating lens secondary fibre cells in 4 dpf sibling (F) and bum-/- mutant (G) zebrafish larvae. In the mutant lenses note the presence of cytoplasm with a much lighter appearance (arrowheads and inset G′), sometimes still containing nuclei (N, inset), embedded in layers of secondary fibre cells that appear to have differentiated normally. Also note the swelling of some of the outermost fibre cells (s), which is however not yet as pronounced as at later developmental stages (see Fig. 4). (H) Lobe-like protrusion of secondary lens fibre cells near the posterior pole of a bum-/- mutant lens. Note that some of the fibre cells, particularly in the outermost layers, appear to be swollen (s). (I) Part of the central retina showing normal development of the layers of the neural retina, the optic nerve and the retinal pigment epithelium. Abbreviations: C, cornea; EC, lens epithelial cells; FC, (primary and secondary) fibre cells; GC, retinal ganglion cells; INL, inner nuclear layer; IPL, inner plexiform layer; L, lens; N, remaining nucleus in a late-stage differentiating secondary lens fibre cell; n, nuclei in a early-stage differentiating secondary lens fibre cells; NR, neural retina; ON, optic nerve; OPL, outer plexiform layer; PFC, primary lens fibre cells; PRC, photoreceptor cells; RPE, retinal pigment epithelium; SFC, secondary lens fibre cells. PHENOTYPE:
|
|
Mutant bum-/- lenses display significantly increased levels of apoptotic cell death in the anterior lens region at 4 dpf. (A, B) External views of the heads (A, B) and eyes (A′, B′) of 4 dpf sibling (wild-type, wt; A, A′) and bum-/- larvae (B, B′) showing Acridine Orange fluorescence to reveal cells undergoing apoptosis . The outer margins of the eyes (outer dotted lines in A and B) and the inner margins of the pupils (inner dotted lines in A and B; dotted lines in A′ and B′) are indicated. Note the reduced and irregular pupil diameter in the bum-/- mutant larvae. While the wild-type lenses do not show Acridine Orange-positive cells (A′), mutant lenses regularly contain numerous fluorescent cells (B). (C) Statistical analysis of the number of apoptotic cells in 4 dpf sibling (left) and bum-/- mutant larvae (right) showing highly significant numbers of apoptotic cells in the mutant lenses (but not in the wild-type), which suggests a mechanism for the regression of the lens tumours in bum-/-. Error bars represent the standard error of the mean (SEM). PHENOTYPE:
|
|
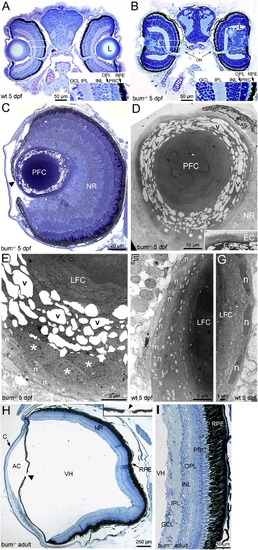
The lens in 5 dpf bum-/- zebrafish shows a severe degeneration of secondary lens fibre cells and can be ectopically located in the neural retina. (A–F) Histology of the eyes in larval (A–G) and adult bum-/- zebrafish (H, I). (A, B) Toluidine blue-stained transversal sections through the central part of the eyes of 5 dpf sibling (wild-type, wt; A) and bum-/- (B) zebrafish larvae. Insets in bottom-right corners show magnifications of the boxed areas in the retinas of the eyes shown on the left in A and B, respectively. (C) Semi-thin section of a bum-/- eye at 5 dpf. The higher magnification of the lens reveals that in the outer lens cortex, the secondary lens fibre cells appear detached from each other with large empty spaces between them (see bracket indicating this region at the lens’ posterior pole). The central lens nucleus, however, seems to be composed of morphologically normal primary lens fibre cells. Note that part of the iris reaches beyond the centre of the lens (black arrowhead) leaving only a small, lopsided pupil opening. (D–G) Thin-section electron microscopy images of 5 dpf bum-/- (D, E) and wild-type lenses (F–G). The images of mutant lenses show that the initial stages of fibre cell differentiation occur normally with the cells (and their nuclei) adopting a flattened shape during cellular elongation. Soon after the onset of differentiation (3–4 cells into the lens), however, the differentiating secondary lens fibre cells appear to swell, increasing in thickness by a factor of up to ten (asterisks). A further 1–2 cells deeper into the lens, the secondary fibre cells appear to detach from each other and/or fragment. This results in large empty, vacuolar spaces (v) between fibre cell fragments. The fibre cells of the lens nucleus by contrast appear to have differentiated normally. Such swelling, detachment and fragmentation of secondary lens fibres is never observed in age-matched wild-type sibling lenses (F, G). (H, I) Morphology of the adult eye in bum-/- as revealed in transverse sections through the central part of the eye. Except for the conspicuous absence of the lens and an almost closed pupil opening (arrowhead and inset in H), the morphology of the eye in bum-/- mutant zebrafish is comparable to that of wild-type individuals, with a neural retina displaying all characteristic layers, a retinal pigment epithelium interdigitating with the well-developed photoreceptor outer segments, as well as a normally formed anterior chamber, vitreous humour cavity and cornea. A higher magnification of part of the retina of an adult bum-/- mutant zebrafish shows normal retinal layering (I). Abbreviations: AC, anterior chamber; C, cornea; EC, lens epithelial cells; GCL, ganglion cell layer; INL, inner nuclear layer; IPL, inner plexiform layer; L, lens; LFC, lens fibre cells; n, nuclei of differentiating secondary lens fibre cells; NR, neural retina; ON, optic nerve exiting the retina; OPL, outer plexiform layer; PFC, primary lens fibre cells; PRC, photoreceptor cells; RPE, retinal pigment epithelium; v, vacuolar spaces; VH, vitreous humour. |
|
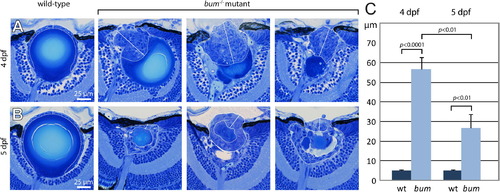
The hyperproliferation of the lens epithelium regresses between 4 and 5 dpf in bum-/- mutant larvae. (A–B) Histological sections of sibling (wild-type, wt) and bum-/- mutant eyes at 4 dpf (A) and 5 dpf (B). The lens epithelial cells are encircled (curved dotted lines) and the lines along which the thickness of the lens epithelium was measured are indicated (straight solid lines). Note that the epithelium in the wild-type lenses always comprises a single layer of cells (epithelial monolayer), whereas in the mutant lenses it can be very significantly expanded (three representative examples are shown for each developmental stage). (C) Quantitative measurements of the thicknesses of wild-type (n = 10) and bum-/- mutant lens epithelia (n = 10 for 4 dpf; n = 9 for 5 dpf). Mutant epithelia are on average approx. 10-fold and 5-fold thicker than wild-type epithelia at 4 and 5 dpf, respectively. Notably, there is a statistically significant decrease of 53% in the thickness between mutant lens epithelia from 4 to 5 dpf. Error bars represent the standard error of the mean (SEM). |
|
bum-/- mutant zebrafish survive to adulthood but show smaller eyes lacking a pupil. (A–D) External views of adult sibling (wild-type; wt) and homozygous bum-/- zebrafish. (A) Lateral views of the left (L) side of the bodies of a female (F) and a male (M) adult sibling zebrafish. (B) Lateral views of the left side of the body of a female and a male homozygous bum-/- adult zebrafish revealing a bilateral reduction in eye size and a complete lack of a pupil in the mutant. (C, C′) Magnifications of the left (L) and right (R) sides of the head of the female homozygous bum-/- zebrafish shown in B. (D, D′) Magnifications of the left (L) and right (R) sides of the head of the male homozygous bumper zebrafish shown in B. Note that adult sibling and bum-/- individuals are of comparable size and, except for the eyes, overall morphology. Scale bars: A, B: 1 cm, C–D′: 2 mm. PHENOTYPE:
|
|
bum-/- zebrafish are severely visually impaired. (A–B) Lateral (A) and dorsal (B) views of adult bum-/- mutant zebrafish and sibling (wild-type) zebrafish swimming together. Note the significantly darker overall appearance of the mutant fish (asterisks; lower individual in A and upper individual in B, respectively) compared to their siblings when kept under bright light conditions. (C–F) Optokinetic response of bum-/- mutants and siblings at 5 dpf (C, D) and 7 dpf (E, F). Both contrast sensitivity (C, E) and visual acuity (D, F) are affected by the mutation. In both paradigms the eye velocity as a measure of visual performance is lower in bum-/- mutant larvae compared to their siblings. The inset in C shows a schematic representation of the experimental set-up. |
|
Skeletal analyses by whole-mount staining reveal craniofacial defects and a normal ocular skeleton. Alizarin red staining, except for G, which is stained with Alcian blue. (A–C) bum-/-, (D–F) wild-type (wt). Comparative linear dimensions are shown and indicated by a–g. (A) dorsal view, bum-/- 30 mm SL; (B) lateral view, bum-/- 30 mm SL; (C) dorsal view after removal of jaws, eyes and opercular complex, bum-/- 21 mm SL; (D) dorsal view, wt 30 mm SL; (E) lateral view, wt 30 mm SL; (F) dorsal view after removal of jaws, eyes and opercular complex, wt 21 mm SL; (G) bum-/- 10 mm SL, Alcian blue-stained; (H) scleral ossicles, bum-/- 30 mm SL; (I) scleral ossicles of wt 21 mm SL, top, and bum-/- 21 mm SL, below; (J) supraorbital bone of wt 21 mm SL, top, and bum-/- 21 mm SL, below; (K) suborbital bone of wt 21 mm SL, top, and bum-/- 21 mm SL, below; (L) lateral bones including pterygoid complex, preopercle, symplectic, and quadrate bones, wt 21 mm SL, top, and bum-/- 21 mm SL, below; (M) hyomandibular, wt 21 mm SL, right, and bum-/- 21 mm SL, left; (N) subopercle, wt 21 mm SL, right, and bum-/- 21 mm SL, left; (O) interopercle, wt 21 mm SL, top, and bum-/- 21 mm SL, below; (P) mandible, wt 30 mm SL; (Q) mandible, bum-/- 30 mm SL; (R) mandible, bum-/- 28 mm SL; (S) mandible, bum-/- 30 mm SL; (T) maxilla complex, bum-/- 30 mm SL. Abbreviations: cb, ceratobranchials; ch, ceratohyal cartilage; en, entopterygoid; mc, Meckel’s cartilage; mx, maxilla; p, palatine; pm, premaxilla; q, quadrate; sc, scleral cartilage; so, scleral ossicles; sy, symplectic. Scale bars: 500 μm unless otherwise indicated. PHENOTYPE:
|
|
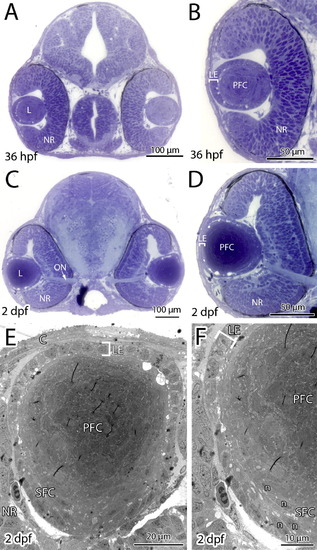
Eye development proceeds normally in bum-/- embryos until 2 dpf. (A–D) Toluidine blue-stained transversal semi-thin sections through the central part of the eyes of 36 hpf (A, B) and 2 dpf (C, D) bum-/- mutant embryos. A and C show cross-sections of entire heads, B and D shows higher magnifications of individual eyes. (E, F) Thin-section electron microscopy images of transversal sections through the central part of the lens of 2 dpf bum-/- zebrafish larvae: overview (E) and higher magnification of the lens’ bow region (F) comprising the early differentiating secondary lens fibre cells. These images demonstrate that the hyperproliferation of the anterior lens epithelium and the degenerative changes in the secondary lens fibre cells observed in bum-/- mutant larvae at later developmental stages are not apparent at these earlier stages. Brackets in panels B and D–F indicate the monolayer of anterior lens epithelial cells. The overall morphology of the head (A, C) and eye (B, D), including the development of the neural retina are also normal in bum-/- mutant larvae. Abbreviations: C, cornea; L, lens; LE, lens epithelium; n, elongated nuclei of the differentiating secondary lens fibre cells; NR, neural retina; ON, optic nerve leaving the eye; PFC, primary lens fibre cells; SFC, secondary lens fibre cells. |
|
Histological section showing the layers of the adult wild-type zebrafish retina. See Fig. 4I for the retinal morphology in adult bum-/- zebrafish. Abbreviations: GC, retinal ganglion cells; INL, inner nuclear layer; IPL, inner plexiform layer; OPL, outer plexiform layer; PRC, photoreceptor cells; RPE, retinal pigment epithelium. |

Unillustrated author statements PHENOTYPE:
|
Reprinted from Mechanisms of Development, 127(3-4), Schonthaler, H.B., Franz-Odendaal, T.A., Hodel, C., Gehring, I., Geisler, R., Schwarz, H., Neuhauss, S.C., and Dahm, R., The zebrafish mutant bumper shows a hyperproliferation of lens epithelial cells and fibre cell degeneration leading to functional blindness, 203-219, Copyright (2010) with permission from Elsevier. Full text @ Mech. Dev.