- Title
-
Reiterative use of the notch signal during zebrafish intrahepatic biliary development
- Authors
- Lorent, K., Moore, J.C., Siekmann, A.F., Lawson, N., and Pack, M.
- Source
- Full text @ Dev. Dyn.
|
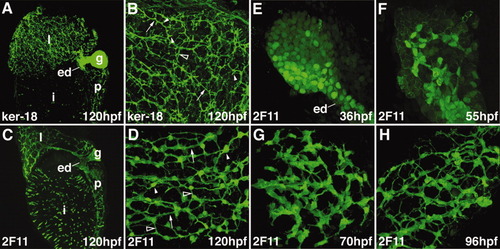
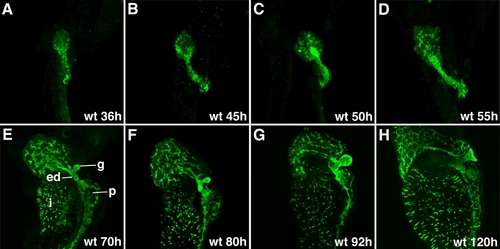
Zebrafish intrahepatic biliary development. A, B: Confocal projections through the liver of a 120-hpf larvae stained with the anti-Keratin 18 antibody. The low-power image (A) shows the intrahepatic biliary network (l), extraehepatic ducts (ed), and gallbladder (g). The high-power image (B) shows that the keratin-18 protein detects 3 classes of intrahepatic ducts: long ducts (arrow), interconnecting ducts (open arrowhead), and terminal ductules (arrowhead). C, D: Confocal projections through the liver of a 120-hpf larvae stained with the 2F11 antibody. The low-power image (C) shows that the 2F11 epitope is presented within the intrahepatic (l) and extrahepatic (ed) ductal systems as well as the gallbladder (g). The high-power image (D) shows that the 2F11 epitope is present in the nucleus of the biliary epithelial cell (arrowhead). This epitope is also detected on the long ducts (arrow) and interconnecting ducts (open arrowhead) but not the terminal ductules. E-H: Developmental pattern of the 2F11 epitope in the intrahepatic biliary system between 36 and 96 hpf. |
|
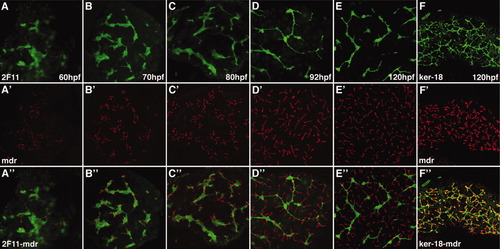
Development of hepatocyte canaliculi and intrahepatic biliary network. Developmental pattern of the 2F11 epitope (A-E) and Mdr epitope, a canalicular transporter (A′-E′) with overlap of the two markers (A″-E″). The length and number of the canaliculi increases between 60 and 120 hpf. The overlap of the two markers shows that each canaliculus develops in close association with the 2F11-positive biliary epithelia. Each canaliculus drains into a single intrahepatic bile duct. The ducts associated with many of the canaliculi in the 120-hpf sample are out of the plane of focus. F, F′, F″: Comparable confocal projections through the liver of a larva immunostained with the keratin-18 and Mdr antibodies. Note overlap (yellow) of the keratin-18 epitope in the terminal ductules with the Mdr protein in the canalicular membrane. |
|
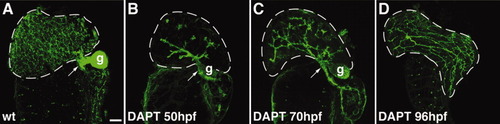
Notch signaling is required for expansion of the intrahepatic biliary ductal network. A-D: Confocal projections through the liver of a control 120-hpf larva and 120-hpf larvae treated with DAPT beginning at 50 hpf (B), 70 hpf (C), and 96 hpf (D) that were stained with the keratin-18 antibody. These images show that at all stages examined, expansion of the biliary network is disrupted when Notch signaling is inhibited by DAPT. Development of the extrahepatic duct (arrows) and gallbladder (g) are not affected by Notch inhibition. These structures are out of the plane of focus in the 96-hpf larva. |
|
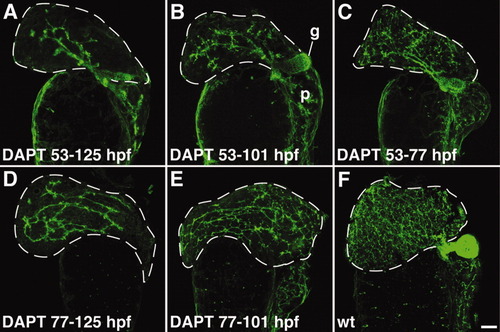
Liver cells respond to the Notch signal throughout larval development. A-F: Biliary expansion resumes upon reinitiation of Notch signaling associated with DAPT withdrawal. There is progressive increase in the complexity of the biliary network in 125-hpf larvae treated with DAPT for shorter intervals beginning at 53 hpf. All images are derived from larvae immunostained with the anti keratin-18 antibody. The gallbladder (g) is out of the plane of focus in D and E. p, pancreas. |
|
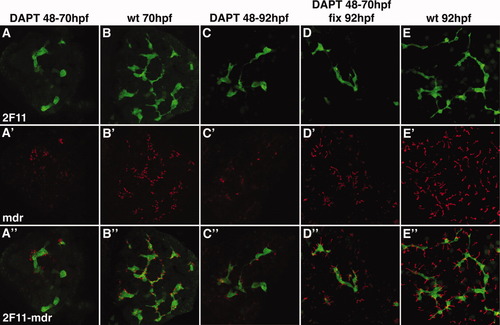
Notch signaling is required for canalicular development. Confocal projections (5 μm) through the liver of a 70-hpf larva (A) and two 92-hpf (C, D) larvae treated with DAPT from 48-70 hpf (A); DAPT from 48-92 hpf (C) and DAPT from 48-70 hpf (D). A 70-hpf (B) and 92-hpf wild type larva (E) are also shown. All larvae were immunostained with Mdr (red) and 2F11 (green) antibodies. DAPT treatment arrests canalicular development (red) and biliary development (green). Canalicular development reinitiates with DAPT withdrawal (D). Note that the depth of these confocal projections is too small to detect reinitiation of duct development upon DAPT withdrawal. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Notch reporter expression in developing intrahepatic biliary cells: Confocal projections through the liver of 45-120-hpf Notch reporter larvae following anti-GFP immunostains. The GFP expression pattern closely resembles the distribution of the 2F11 epitope within intrahepatic biliary cells. |
|
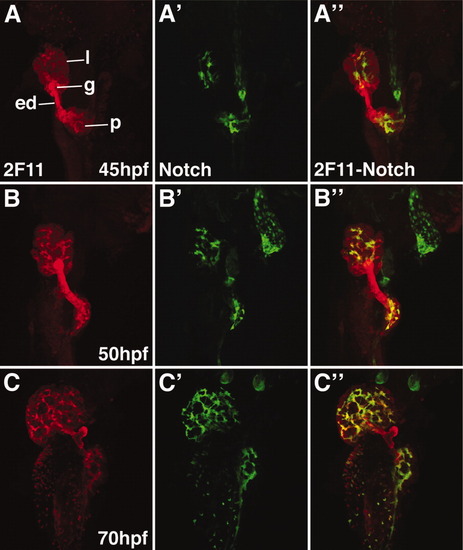
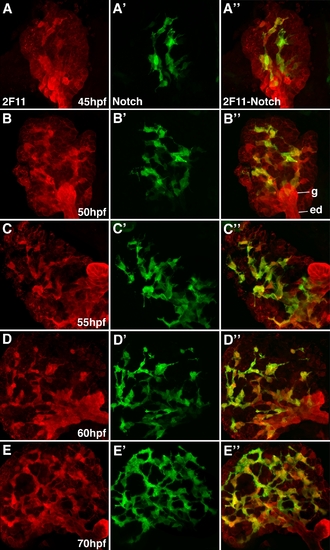
Notch reporter expression overlaps with the 2F11 epitope in developing intrahepatic biliary cells. Whole mount confocal images through the liver of developing Notch reporter larvae stained with the 2F11 antibody (A-C) and a GFP antibody (A′-C′) with overlap of the two markers (A″-C″). GFP-positive biliary epithelia are first detected at 45 hpf (A′). At this stage, there is strong 2F11 expression in the gallbladder (g), extrahepatic duct (ed), and in a few liver parenchymal cells (l). Only partial overlap between GFP and 2F11 is seen in the liver at this stage. GFP-positive cells are detected in the pancreas. From 50-70 hpf, there is a progressive increase in the number of GFP-positive cells. At 50 hpf, there is significant overlap between the GFP and 2F11 epitopes in the biliary cells. At 70 hpf, there is nearly complete overlap between these markers in the biliary cells. The gallbladder and extrahepatic duct remain GPF-negative at all stages. The pancreatic ductal network is also GFP-positive (p). |
|
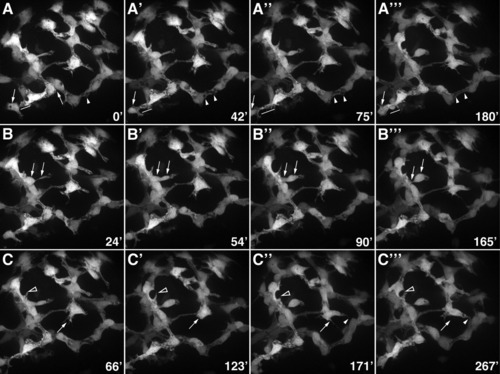
Morphogenesis of the intrahepatic biliary network. Confocal projections through the liver that was dissected from a 76-hpf Notch reporter larva and cultured for 4.5 hr. Numbers in the bottom right corner indicate duration of culture in minutes. A: These images show progressive lengthening and then shortening of the cytoplasmic bridge between two adjacent biliary cells (arrows). Fusion of cytoplasmic vesicles leading to the formation of a ductal lumen within two adjacent cells is also evident (arrowheads). B: These images show the migration of a single biliary cell via a cytoplasmic extension (arrows). C: These images show the dynamic pattern of filopodia extension and retraction from two biliary cells (arrows and arrowheads). |
|
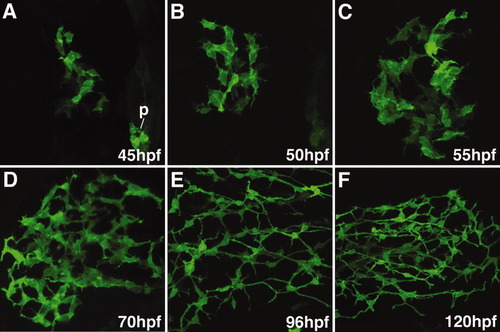
Development of the intrahepatic and extrahepatic biliary system: Confocal projections through the liver of embryos and larvae immunostained with the 2F11 antibody. These images outline liver morphogenesis and development of the gallbladder (g) and extrahepatic ducts (ed) in relation to the intrahepatic ductal network. The 2F11 epitope is also detected in secretory cells on the intestinal epithelium (i), the ductal network of the pancreas (p). |
|
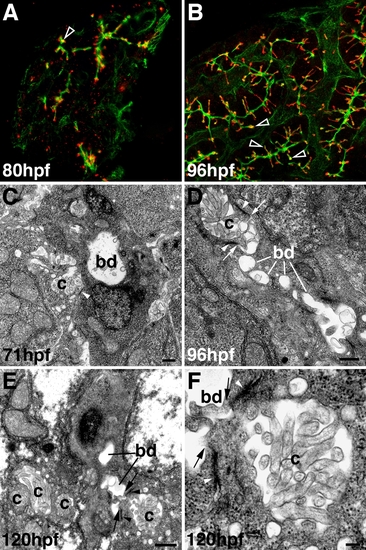
Development of hepatocyte canaliculi and intrahepatic biliary network. A, B: Confocal projections through the liver of an 80-hpf (A) and 96-hpf (B) larva immunostained with Keratin-18 (green) and Mdr (red) antibodies. Compared with double immunostains of 5-dpf larvae (Fig. 2F″), there is much less overlap of the Mdr-1 and Keratin-18 epitopes at these developmental stages (open arrowheads). C-F: Transmission electron micrographs showing the canalicular-terminal duct junction (arrows) in 71-hpf (C), 96-hpf (D), and 120-hpf (E, F) larvae. bd, bile duct; c, canaliculus; arrowhead, tight junctions. Bar = 500 nm in C-E, 100 nm in F |
|
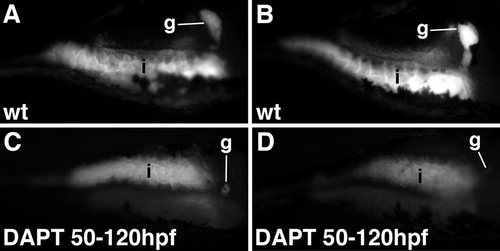
Notch inhibition reduces biliary secretion. A-D: Fluorescence microscopy images (lateral view) of live 6-dpf larvae (144 hpf) following ingestion of the fluorescent lipid bodipy-C16. In wild types (wt), strong fluorescence is detected in the intestine (i) and gallbladder (g). Fluorescence emission from both tissues is reduced in larvae treated with DAPT between 50-120 hpf. Each panel shows a unique larva. These data are representative of 10 wild type and 10 DAPT-treated larvae. |
|
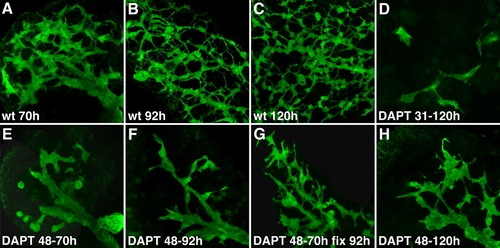
Notch signaling is required for expansion of the intrahepatic biliary ductal network. A-C: Confocal projections through the liver of wild type larvae (70, 92, and 120 hpf) and larvae treated with DAPT from 48-70 hpf (E), 48-92 hpf (F), 31-120 hpf (D), and 48-120 hpf (H), fixed immediately after the treatment, followed by 2F11 immunostainings. A larva treated with DAPT from 48-70 hpf and then fixed at 92 hpf after DAPT withdrawal at 70 hpf is also shown (G). DAPT treatment arrests intrahepatic biliary development (compare A with E; B with F; C with D and H). There is expansion of the ductal network after DAPT withdrawal (compare G with F). |
|
Notch reporter expression in developing intrahepatic biliary cells: Whole mount confocal images (63x) through the liver of developing Notch reporter larvae stained with the 2F11 antibody (A-D) and a GFP antibody (A′-D′) with overlap of the two markers (A″-D″). GFP-positive biliary epithelia are first detected at 45 hpf (A′). At this stage, scattered 2F11-positive cells are present in the liver with only partial overlap with GPF. From 50-70 hpf, there is progressive increase in the number of GFP-positive cells. AT 50 hpf, there is significant overlap between the GFP and 2F11 epitope in the biliary cells. There is nearly complete overlap between these markers at 60 and 70 hpf. The gallbladder (g) and extrahepatic duct (ed) remain GPF negative. |