- Title
-
Suppression of Alk8-mediated Bmp signaling cell-autonomously induces pancreatic β-cells in zebrafish
- Authors
- Chung, W.S., Andersson, O., Row, R., Kimelman, D., and Stainier, D.Y.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
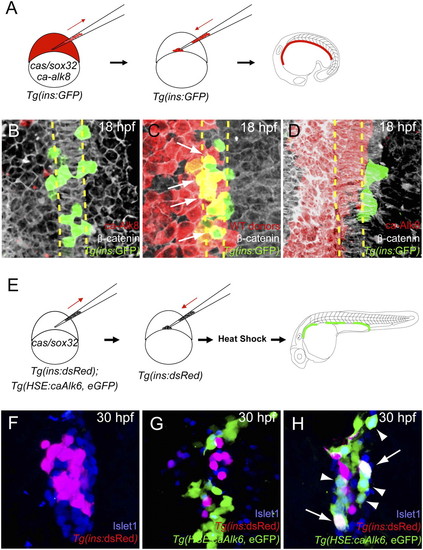
Activation of Bmp signaling cell-autonomously blocks the induction of dorsal bud-derived endocrine cells. (A) Schematic diagram of the cell transplantation protocol. Tg(ins:GFP) donors were injected with cas/sox32 and ca-alk8 RNA, along with rhodamine dextran, and the cells were transplanted into Tg(ins:GFP) hosts. (B–D) Ventral confocal images of Tg(ins:GFP) (green), β-catenin (white), and rhodamine dextran (red) at 18 hpf (the notochord is outlined by yellow dashed lines). (B and C) Tg(ins:GFP)-expressing cells are normally located close to the notochord in wild type (B) and hosts containing control donor cells (C). (D) Tg(ins:GFP) expression was absent in ca-Alk8-expressing endodermal cells. (E) Schematic diagram of the cell transplantation protocol. cas/sox32 overexpressing Tg(ins:dsRed); Tg(HSE:caAlk6, eGFP) donor cells were transplanted into Tg(ins:dsRed) hosts, and subsequently heat-shocked at various time points. (F–H) Confocal projection images of hosts with Tg(HSE:caAlk6, eGFP)-expressing cells (green) at 30 hpf after staining for dsRed (red) and Islet1 (blue), comparing control (F) and hosts [heat shock was applied at 9 (G) or 11 hpf (H)]. (G) When ca-alk6 expression was induced at 9 hpf, the number of Tg(ins:dsRed) and Islet1-expressing cells was reduced (compare F and G), and the Tg(HSE:caAlk6, eGFP)-expressing cells failed to express endocrine markers. (H) When ca-alk6 expression was induced at 11 hpf, several Tg(HSE:caAlk6, eGFP)-expressing cells expressed Islet1 (arrowheads) and some of them also coexpressed Tg(ins:dsRed) (arrows). |
|
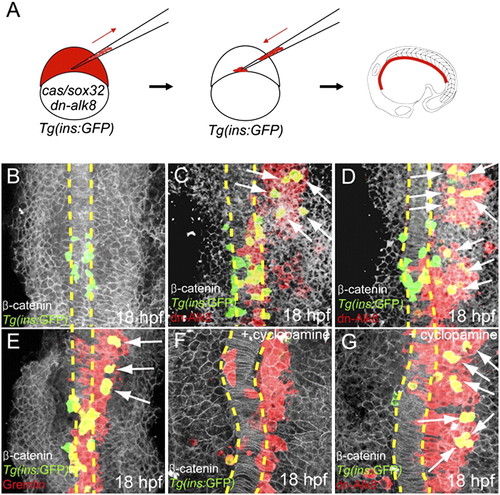
Suppression of Bmp signaling is sufficient to induce ectopic dorsal bud-derived pancreatic β-cells cell-autonomously. (A) Schematic diagram of the cell transplantation protocol. Tg(ins:GFP) donors were injected with cas/sox32 and dn-alk8 RNA along with rhodamine dextran (red), and the cells were transplanted into Tg(ins:GFP) hosts. (B–E) Ventral confocal images of Tg(ins:GFP) (green), β-catenin (white), and rhodamine dextran (red) at 18 hpf (the notochord is outlined by yellow dashed lines). (B) In control embryos, Tg(ins:GFP)-expressing cells are located close to the notochord. (C and D) Ectopic Tg(ins:GFP)-expressing cells (arrows) were found in lateral and anterior endodermal regions where dn-Alk8-expressing cells had incorporated, and all these ectopic cells were donor derived. (E) Gremlin1a overexpression in the endoderm also resulted in the ectopic formation of Tg(ins:GFP)-expressing cells (arrows). (F) When Hedgehog signaling was blocked with cyclopamine, Tg(ins:GFP)-expressing cells were almost absent in hosts containing control donor cells. (G) dn-Alk8 expression still induced the formation of Tg(ins:GFP)-expressing cells (arrows) even after cyclopamine treatment, and it did so cell-autonomously. |
|
Activation of Bmp signaling cell-autonomously blocks the induction of ventral bud-derived endocrine cells. (A–C) Confocal images of Tg(gutGFP) (green) with mRNA expression (red) of bmp4 (A), gremlin1a (B), and noggin1 (C) at 60 hpf. (D and E) Confocal images of wild-type endoderm showing β-catenin (white) and Id2 (red) expression at 72 hpf. (D) Id2 expression is excluded from the hepatopancreatic duct cells (HPD; yellow dashed line) and appears to be downregulated or excluded from the pancreatic endocrine cells (PI, principal islet; black dashed line). (E) Id2 expression is also excluded from the intrapancreatic duct cells, labeled by Tg(-3.5nkx2.2a:GFP) expression (41) (green; arrows). (F) Confocal image of wild-type endoderm showing Tg(gutGFP) (green) and Islet1 (red) expression at 72 hpf. The ventral bud-derived endocrine cells (arrows) can be found at the junction between the pancreas and hepatopancreatic duct [the principal islet (PI) is outlined by black dashed line]. (G) Schematic diagram of the cell transplantation protocol. cas/sox32-overexpressing Tg(HSE:caAlk6, eGFP) donor cells were transplanted into wild-type hosts. Hosts were heat-shocked at 50 hpf and fixed at 72 hpf. After applying heat shock, hosts were treated with the Fgf receptor inhibitor SU5402, which induces ectopic Islet1-positive cells in the hepatopancreatic duct. (H) Single plane image of embryo stained for GFP (green), Pan-cadherin (white), and Islet1 (red) showing that Tg(HSE:caAlk6, eGFP)-expressing cells (arrows) fail to express Islet1 and display clear segregation from the ectopic Islet1-expressing cells (asterisks). (I and J) Effect of cell-nonautonomous activation of Bmp signaling by heat shock of Tg(hsp70l:bmp2b) at 50 hpf and subsequent treatment with SU5402 until fixation. Confocal projections of embryos stained for Pan-cadherin (white) and Islet1 (red) comparing control (I) and experimental (J) embryos. (I) Upon SU5402 treatment, ectopic Islet1-positive endocrine cells appeared in the hepatopancreatic duct (yellow dashed area), an effect that can be partially blocked by overexpression of Bmp2b (J). (K) Diagram of the endodermal organs at 72 hpf showing the liver (L; light red), pancreas (P; green), hepatopancreatic duct (HPD; yellow), gall bladder (GB; light yellow), and intestinal bulb (IB), as well as the ventral bud-derived endocrine cells (VBE; red) and principal islet (PI; red). |
|
Suppression of Bmp signaling in a cell-autonomous fashion leads to an increase in ventral bud-derived endocrine cells. (A and B) Confocal projection of control (A) and alk8 mutant (B) endoderm at 56 hpf. Tg(ins:dsRed) embryos were stained for Pan-cadherin (white). (B) alk8 mutants exhibit a very small ventral pancreatic bud (arrow). (C and D) Confocal projections of control (C) and alk8 morphant (D) Tg(ptf1a:GFP);Tg(ins:dsRed) embryos stained for Pan-cadherin (white). In alk8 morphants (D), the ventral pancreas buds out but fails to expand and engulf the principal islet. (E) Schematic diagram of the cell transplantation protocol. Tg(gutGFP) donors were injected with cas/sox32 mRNA and alk8 splice-blocking MO, and the donor cells were transplanted into wild-type hosts. (F and G) Confocal projections of hosts containing alk8 morphant donor cells (green) stained for Pan-cadherin (white) and Islet1 (red) at 56 (F) and 72 (G) hpf. Most alk8 morphant donor cells gave rise to Islet1-expressing endocrine cells (arrows). (H and I) Confocal projections of Tg(ins:GFP);Tg(ins:dsRed) embryos stained for Pan-cadherin (white) comparing control (H) and dorsomorphin-treated (I) embryos. Compared with control embryos (H), dorsomorphin-treated embryos showed an increased number of GFP-only-positive β-cells (arrows) (I). |
|
Control donor cells gave rise to pancreatic endocrine cells in host embryos without displaying an increased preference for an endocrine cell fate. (A and B) Confocal images of host embryos containing control donor cells (rhodamine dextran, red) at 30 hpf. Control donor cells incorporated into Islet1 (blue)-expressing pancreatic endocrine cells, and some of them differentiated into Tg(ins:GFP)-expressing pancreatic β-cells (green; triple-labeled cells appear white). For clarity, the red color is excluded in the corresponding Left panel (A). |
|
Genes encoding Bmp ligands and antagonists are expressed in the mesenchymal cells adjacent to the foregut endoderm between 50 and 72 hpf. (A–L) Confocal images of Tg(gutGFP) expression (green) with mRNA expression (red) of bmp2b (A–C), bmp4 (D–F), gremlin (G–I), noggin1 (J and K), and bmp6 (L) at 50 (A, D, G, and J), 60 (B, E, H, K, and L), and 72 (C, F, and I) hpf. |
|
Expression of Id2 is excluded from the intrapancreatic duct cells. (A) Confocal image of Tg(-3.5nkx2.2a:GFP) expression (green), which strongly marks the intrapancreatic duct (IPD) cells and weakly marks the extrapancreatic duct (EPD) cells and endocrine cells adjacent to the principal islet (PI) at 72 hpf. β-Catenin (white) outlines the general morphology of the pancreas. (B–E) Id2 (red) is expressed in acinar cells but appears to be downregulated or excluded from the intrapancreatic duct cells (green; arrows) in distal (B and C) and proximal (D and E) regions of the pancreas. For clarity, the green color is excluded in the corresponding Right panels (C and E). |
|
Verification of the alk8 splice-blocking morpholino by comparing alk8 mutants with alk8 morphants, and RT-PCR. (A–D) Morphology of the tail at 30 hpf. (A and C) Wild-type and alk8 heterozygote embryos display normal tail development. (B) alk8 mutants display a dorsalized phenotype resulting in a lack of the ventral tail fin. (D) All embryos injected with the alk8 morpholino exhibit ventral tail fin defects (75% of the embryos lacked the whole ventral fin and 25% had a partial reduction of the fin). (E) RT-PCR showing reduced amount of spliced alk8 mRNA in embryos injected with alk8 morpholino at 30, 56, and 72 hpf. Twenty embryos from each condition and stage were used for cDNA synthesis. The alk8 morpholino blocks the splice donor site of the second coding exon, leading to a decreased PCR product because the primers used anneal to the second (52-ctgctagtcatgtggtagaatgctg-32) and fifth (52-cacttcaccgtaccgtcctt-32) coding exons. Equal amounts of cDNA template is indicated by amplification of elongation factor 1-α, using the primers (52-tcaccctgggagtgaaacagc-32) and (52- acttgcaggcgatgtgagcag-32). |
|
alk8 splice-blocking morpholino-injected donor cells behave like control cells and can give rise to dorsal bud-derived endocrine cells. (A) Schematic diagram of the cell transplantation protocol. Tg(ins:GFP) donors were injected with cas/sox32 mRNA and alk8 MO along with rhodamine dextran, and the cells were transplanted into Tg(ins:GFP) hosts. (B and C) Ventral confocal images of Tg(ins:GFP) (green), β-catenin (white), and rhodamine dextran (red) at 18 hpf (the notochord is outlined by yellow dashed lines). The donor cells (red) did not give rise to ectopic Tg(ins:GFP)-expressing cells, because maternally derived Alk8 is present during early stages. |
|
Some ectopic endocrine cells induced by mosaic knockdown of alk8 send out long protrusions. (A–B′) Confocal projections of hosts containing alk8 morphant donor cells (Tg(gutGFP); green) stained for Pan-cadherin (white) and Islet1 (red) at 56 (A and A′) and 72 (B and B′) hpf. alk8 morphant donor cells gave rise to Islet1-expressing endocrine cells in nonpancreatic tissues, such as the pharyngeal endoderm and intestine (yellow dashed squares). These ectopic endocrine cells appear to send out long protrusions, possibly in an attempt to connect with the principal islet (PI). For clarity, A′ and B′ show a magnified view of the cells marked by the yellow dashed squares in A and B, respectively. Also, the white color is excluded in the corresponding Right panels. |
|
Mosaic knockdown of alk8 preferentially generates additional Insulin and Somatostatin-expressing cells. (A) Schematic diagram of the cell transplantation protocol. Tg(gutGFP) donor embryos were injected with cas/sox32 mRNA and alk8 splice-blocking MO before transplantation into wild-type hosts. (B–D) Confocal images of hosts containing Tg(gutGFP) donor cells (green) and stained for Insulin (B), Glucagon (C), or Somatostatin (D) (red) at 72 hpf. (E) Proportional distribution of hormone content of alk8 morphant cells located in the islet. The percentages were calculated by counting the number of donorderived hormone-expressing cells and dividing it by the total number of the donor cells in the islet (total number of cells counted = 306). Host embryos were stained for Insulin or Glucagon/Somatostatin; because the sum of the percentages of Insulin, Glucagon, and Somatostatin-expressing cells did not reach 100%, the data indicate that some donor cells differentiated into other endocrine cell types (e.g., ε- or γ-cells), or nonendocrine cells. |
|
Mosaic knockdown of alk8 cell-autonomously generates ventral bud-derived β-cells. (A) Schematic diagram of the cell transplantation protocol. Tg (ins:GFP);Tg(ins:dsRed) donors were injected with cas/sox32 mRNA and alk8 splice-blocking MO along with Alexa Fluor 647 dextran (blue), and the donor cells were transplanted into Tg(ins:GFP);Tg(ins:dsRed) hosts. (B–C′) Confocal projections of Tg(ins:GFP)- and Tg(ins:dsRed)-expressing islets containing control (B and B′) and alk8 morphant (C–D′) donor cells (blue) at 60 hpf. Control donor cells incorporated into Tg(ins:GFP)- and/or Tg(ins:dsRed)-expressing β-cells without showing any preference. In contrast, alk8 morphant donor cells (blue) gave rise to many GFP-only–positive β-cells (arrows). For clarity, the green color is excluded in the corresponding Left panels (B–D). |