- Title
-
Her6 regulates the neurogenetic gradient and neuronal identity in the thalamus
- Authors
- Scholpp, S., Delogu, A., Gilthorpe, J., Peukert, D., Schindler, S., and Lumsden, A.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
The neurogenetic gradient in fish. Glutamatergic neurogenesis spreads in a wave from posterior to anterior in the developing thalamus. Analysis of the dynamic expression of proneural genes during the development of the thalamic complex by in vivo imaging of double transgenic zebrafish and double in situ hybridisations. Upon induction of shh:GFP in the MDO, neurog1:RFP is induced first in the posterior Th (A, arrowheads). Over time, the caudal thalamus is filled with neurog1 positive cells (D and G). At the 20-somite stage, neurog1 mRNA can be detected within the thalamic complex (B). Over time, the expression increases from posterior to anterior and fills the entire cTh at 33 hpf (E and H). At 20 somite stage, her6 expression marks the entire thalamic complex (B and C) and is gradually down-regulated in the caudal part over time. her6 expression is maintained in the PTh, MDO, rTh and absent from the cTh at 33 hpf (H and I). The expression domains of her6 and neurog1 are abutting. In contrast to neurog1, the proneural gene ascl1a is induced from ventral to dorsal within the her6 positive PTh and rTh from the 20-somite stage (C), to 24 hpf (F) and 36 hpf (I). Embryos are shown laterally, white double-headed arrows indicate the increasing width of the cTh. III, third brain ventricle; cTh, caudal Thalamus; MDO, mid-diencephalic organizer; PTh, prethalamus; rTh, rostral Thalamus; ss, somite stage. EXPRESSION / LABELING:
|
|
Her6 is required to repress the proneural gene neurogenin1. In vivo analysis of the loss of Her6 function in double transgenic zebrafish embryos by confocal microscopy. Knock-down of her6 (her6MO) leads to an increase of neurog1 expression in the thalamic complex at 27 hpf (62/86; A and B). At 33 hpf, lack of Her6 function leads to a massive up-regulation of neurog1 in the entire thalamic complex including PTh and MDO (72/91; C and D). The Shh positive MDO is fragmented. Up-regulation of neurog1 leads to a down-regulation of markers, such as dlx4/6:GFP in the PTh at 36 hpf (53/78; E and F). Similarly, the expression of the rTh marker tal1:GFP is completely lacking in her6 morphant embryos 42 hpf (23/28; G and H). By delivering the her6 MO antisense oligomers into a few cells of the rTh by electroporation, neurog1 expression was induced cell-autonomously, as shown by the expression of RFP tagged with a nuclear localisation sequence (5/11; J, higher magnification of the electroporated cell clone marked by arrowheads in J′). Electroporation of a control MO showed no effect (I and I′). III, third brain ventricle; ACeV, anterior cerebral vein; cTh, caudal thalamus; PTh, prethalamus; PTec, pretectum; rTh, rostral thalamus. |
|
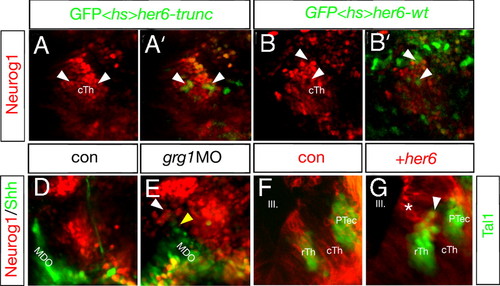
Maintenance of Her6 is required of fate of the rTh. Mis-expression of her6 represses neurog1 expression and activates rostral thalamic fate. Activation of a heat-shock inducible construct driving her6 and the lineage tracer GFP leads to the cell-autonomous down-regulation of neurog1:RFP at 33 hpf (9/15; B and B′; arrowheads). If Her6 lacks the Groucho interaction motif WRPW, the construct does not alter neurog1:RFP expression in a control experiment and shows a co-localization of GFP and nuclear-localized RFP (8/12; A and A2; arrowheads). Knock-down of grg1 resembles the her6 morphant phenotype (23/43; D and E) and leads to an increase in the caudal expression of neurog1:RFP (white arrowhead) as well as a decrease of shh:GFP in the MDO (yellow arrowhead) at 30 hpf. Clonal overexpression of her6 and the membrane-bound red lineage marker mCherry in the cTh leads to the activation of the GABAergic rTh marker tal1:GFP (14/21; I; arrowhead). Notably, dividing cells at the ventricular surface are only positive for the mCherry lineage marker (asterisk) but become tal1:GFP positive after radial migration (arrowhead). Cells containing the lineage tracer only do not express Tal1 (H). III, third brain ventricle; cTh, caudal thalamus; PTec, pretectum; PTh, prethalamus; MDO, mid-diencephalic organizer; rTh, rostral thalamus. EXPRESSION / LABELING:
|
|
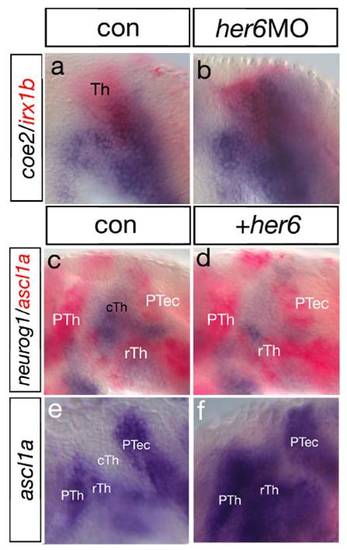
Her6 acts to genetically suppress neurog1. Analysis of ascl1a and irx1b, a pan-thalamic marker, at 30 hpf (A–D) and vglut2.1 and GAD65/67 at 48 hpf (E–H) in her6 and neurog1 morphant embryos. Knock-down of her6 leads to the down-regulation of ascl1a (45/61; A and B) as well as GAD65/67 (22/35; E and F) in the rTh (arrowheads) and in the PTh. Knock-down of neurog1 leads to an unaltered PTh but an increase in width in ascl1a expression (22/34; C) as well as GAD65/67 expression (12/21; G) in the rTh (yellow arrows). Compared to the her6 single knock-down, double knock-down of her6 and neurog1 rescues the prethalamic as well as the rostral thalamic expression domain (ascl1a: 17/22; GAD: 8/17; D and H). Analysis of the MDO in shh:GFP transgenic animals co-expressing ubiquitously the membrane-bound fluorophore mCherry (I–L): Down-regulation of her6 leads to disintegration of the Shh positive MDO (20/26; I and J; arrowheads). In embryos knocked-down for neurog1, the MDO seems unaltered in extension and width compared to the control (8/10; K, arrowhead). Double knock-down of her6 and neurog1 rescues the extend of the shh:GFP expression within the MDO (6/8; L, arrowhead). III, third ventricle; cTh, caudal thalamus; MDO, mid-diencephalic organizer; PTh, prethalamus; rTh, rostral thalamus. |
|
The neurogenetic gradient. Markers of progressive glutamatergic neurogenesis are expressed in a wave from posterior to anterior in the developing thalamus. The neurogenetic marker deltaA (dla) starts in the ventro-posterior sector of the thalamus (marked by irx1b), and gradually fills the thalamus by 30 hpf (a, d, and g, arrows). This wave of expression is followed by that of the terminal neuronal marker vglut2.1, again on the background of the pan-thalamic marker irx1b (b, e, and h). Wnt8b is one of the earliest markers of the MDO and its broad expression at the 20-somite stage (c) is refined to a small band located at theMDOat 24 hpf (f) and 36 hpf (i). At 48 hpf (long-pec stage), her6 is expressed in the PTh, shh positive MDO, and the rTh. The expression domain of neurog1 marks the cTh and is not expressed in the rTh (k). Similar to her6 and complementary to neurog1, the expression domain of ascl1a in the PTh, in the rTh including a lateral part (l), which will give rise to the reticular nucleus) and a medial part [m, which will give rise to parts of the anterior thalamic nucleus; Mueller T, Guo S (2009) The distribution ofGAD67-mRNAin the adult zebrafish (teleost) forebrain reveals a prosomeric pattern and suggests previously unidentified homologies to tetrapods. J Comp Neurol 516:553–568] and in the pretectum at 48 hpf (l). Embryos are shown laterally (a–i), whereas embryos were sectioned horizontally and displayed dorsally in (j–l). White double-headed arrows marks the width of the caudal thalamus (a, b, d, e, g, and h) and the shrinking domain of the rostral thalamus marked by her6 in (c, f, and i). cTh, caudal thalamus; MDO, mid-diencephalic organizer; PTec, pretectum; PTh, prethalamus; rTh, rostral thalamus. |
|
Early expression patterns in the diencephalon. Her6 is expressed in the entire anterior neural plate at tailbud stage (a). The expression domain overlaps with the marker of the posterior forebrain wnt8b shown by a dorsal view. A fluorescent analysis shows that both markers overlap in the area of the posterior diencephalon, shown by quenching of the red fluorescence (a′ and a′′). At the 5-somite stage, her6 is detectable in the telencephalon and in the posterior diencephalon, shown by co-localization with wnt8b in a lateral view (b). At the 10-somite stage, her6 is expressed in the entire thalamic complex including the thalamus anlage marked by irx1b in a lateral view (c). At the 24 hpf stage, expression of the proneural gene neurog1 (d) is first observed in the ventro-posteriro doemain of the irx1b+ positive Th. Notably, we find neurog1 in the basal plate outside to the alar located MDO (asterisk). ascl1b marks the Pth, the rTh and the PTec at 33 hpf (e). Fb, forebrain; Mb, midbrain; PTh, prethalamus; rTh, rostral thalamus; tel, telencephalon; Th, thalamus. |
|
Her6 inhibits expression of the proneural gene coe2 and neurog1. Analysis of Her6 function by double ISH. Knock-down of her6 leads to an increase of coe2 expression in the thalamic complex at 30 hpf (a and b). A double in situ hybridization analysis shows that mis-expression of her6 mRNA leads to the down-regulation of neurog1 expression in the thalamus at 24 hpf (c and d; black dashed line). Expression of ascl1a in the ventral part is increased (yellow dashed line). Subsequently, ascl1a expression is increased upon her6 mis-expression at 30 hpf (22/35; f and g). cTh, caudal thalamus; PTh, prethalamus; rTh, rostral thalamus; Th, thalamus. |
|
Neurog1 represses GABAergic cell fate. Mis-expression of neurog1 mRNA leads to a strong down-regulation of ascl1a expression at 28 hpf (a and b) and a down-regulation of the expression of gad1 at 32 hpf (c and d). neurog1 mRNA over-expression leads gross changes in head morphology such as the lack of optic vesicle formation. Clonally over-expression of neurog1 mRNA leads to the cell-autonomous down-regulation of shh:GFP within the MDO and tal1:GFP within the rTh (32/45; f and h; arrowheads). Control cells carrying the lineage tracer rhodamine dextran only show co-localization with the GFP markers (e and g; arrowheads). cTh, caudal thalamus; MDO, mid-diencephalic organizer; PTh, prethalamus; PTec, pretectum. |
|
Shh controls the timing of proneural gene induction within the Th. At the 10-somite stage, application of the Shh agonist purmorphamine leads to the strong activation of neurog1:RFP (a and b). Conversely, application of the Shh antagonist cyclopamine leads to the severe down-regulation of neurog1:RFP in the caudal thalamus (c). Analysis of smu mutant embryos shows a strong down-regulation of the proneural genes neurog1 and ascl1a in the entire thalamic complex at 33 hpf (d, e, g, and h). Asterisks mark an independent neurog1 expression domain within the basal MDO territory. At the same stage, her6 expression is still detectable in the diencephalon of smu mutant embryos, suggesting a Shh independent maintenance mechanism for her6 mRNA (f and i). cTh, caudal thalamus; MDO, mid-diencephalic organizer; PTec, pretectum; PTh, prethalamus; rTh, rostral thalamus; Tec, Tectum. |