- Title
-
Pivotal Advance: Pharmacological manipulation of inflammation resolution during spontaneously resolving tissue neutrophilia in the zebrafish
- Authors
- Loynes, C.A., Martin, J.S., Robertson, A., Trushell, D.M., Ingham, P.W., Whyte, M.K., and Renshaw, S.A.
- Source
- Full text @ J. Leukoc. Biol.
|
Morphological features of apoptosis are seen during time-lapse analysis of neutrophilic inflammation in zebrafish. (A) Four hours following transection of the tailfin under Tricaine anesthesia, a 4-dpf zebrafish larva was re-anesthetized and mounted in 1% low melting-point agarose containing 0.02% Tricaine. During this time-lapse series (showing a single confocal slice), a neutrophil is seen to cease movement and assume a rounded shape. This is followed by loss of GFP fluorescence. Times since initiation of time-lapse are shown (h:m:s.ms). The images have been selected to be representative of the series, which can be seen in full in Supplemental Movies 1 and 2. Photomicrographs were taken with a x40 Plan NeoFluar oil immersion objective, NA1.3 (Zeiss). As a result of the thickness of the tissue section here, the individual cells are not visible on DIC images. Exposure times were minimized, and there was no evidence of any adverse effects over 16 h of imaging. Similar events are seen under wide-field time-lapse microscopy. (B and C) A similar event occurring in the tailfin enables imaging under DIC of the persisting neutrophil cell "corpse." (B) A fluorescent neutrophil showing features of apoptotic morphology in the tailfin during inflammation resolution. (C) The same neutrophil, a single frame (30 s) later, showing that the neutrophil corpse persists, and the GFP signal has been lost completely. |
|
Dual staining for neutrophil-specific mpx and DNA nick-ends (TUNEL) provides biochemical confirmation of neutrophil apoptosis during resolution of neutrophilic inflammation in unmanipulated zebrafish embryos. Seven dpf zebrafish were injured and fixed 12 h later in 4% paraformaldehyde at 4°C. Neutrophils were revealed by (A) FITC-TSA staining (wild-type fish) for peroxidase activity or (B) immunostaining for GFP (mpx:GFP fish), in combination with TUNEL staining with the ApopTag Red kit. Dual-positive cells representing apoptotic neutrophils can be seen in the right panel of A and B. The images shown are maximum-intensity projected images of at least 20 sections in the Z dimension. Images were taken using a x60 Plan Apo Oil immersion NA1.40 (Olympus). Colocalization of fluorescence in single sections can be seen in Supplemental Movie 3. (C) Larvae at 10 dpf were injured as described previously and treated with the compounds indicated. At 12 h after injury, larvae were fixed and stained for colocalization of neutrophil (FITC-TSA) and apoptotic (TUNEL) markers as above. Dual-positive cells and total neutrophil count were scored from confocal images. Data shown are percent neutrophils costaining with TUNEL markers (i.e., apoptotic neutrophils). P < 0.05 for all treatments compared with DMSO control by one-way ANOVA with Dunnett’s post-test correction. zVAD.fmk, zVD.fmk. |
|
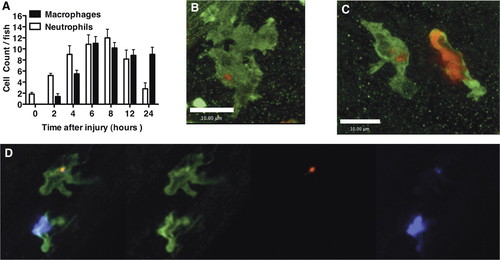
Macrophages take up apoptotic neutrophils during inflammation resolution in vivo. (A) Three dpf mpx:GFP zebrafish were injured as described previously. Two hours before the time indicated, larvae were stained with neutral red for 2 h. The number of neutral red-positive macrophages and GFP-positive neutrophils was counted for each fish under bright-field illumination and 488 nm fluorescent illumination, respectively. (B and C) Seven dpf wild-type zebrafish were injured and fixed 12 h later in 4% paraformaldehyde at 4°C, followed by staining for mpx activity and L-plastin immunohistochemistry, as described in Materials and Methods. Single macrophages can be seen containing TSA-positive neutrophilic material. (C) A neutrophil is shown alongside for comparison. (D) Cy5-TSA staining (purple) was combined with L-plastin staining (green) and TUNEL staining (red) to show colocalization of apoptotic markers and neutrophil markers within a macrophage. Three-dimensional reconstructions are shown in Supplemental Movies 4 and 5 to confirm location of the Cy-3 TSA-positive material completely within the macrophage. Images were taken using a x60 Plan Apo Oil immersion NA1.40 (Olympus). |