- Title
-
Notch Signaling Functions as a Cell-Fate Switch between the Endothelial and Hematopoietic Lineages
- Authors
- Lee, C.Y., Vogeli, K.M., Kim, S.H., Chong, S.W., Jiang, Y.J., Stainier, D.Y., and Jin, S.W.
- Source
- Full text @ Curr. Biol.
|
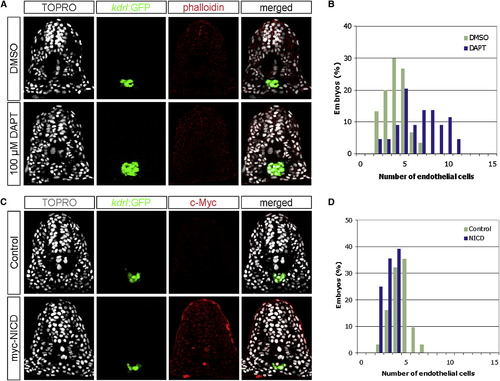
Notch Signaling Negatively Regulates the Number of Endothelial Cells during Zebrafish Development (A) Transverse sections of 18 hpf embryos, DMSO- or DAPT-treated, visualized for TOPRO (white), kdrl:GFP (green), and phalloidin (red). (B) Quantification of endothelial nuclei per focal plane in DMSO-treated (n = 30) or DAPT-treated (n = 44) embryos. (C) Transverse sections of 18 hpf phenotypic wild-type siblings and Tg(hsp70l:Gal4)kca4;Tg(UAS:myc-NICD)kca3 embryos visualized for TOPRO (white), kdrl:GFP (green), and c-Myc as a surrogate measure for NICD expression (red). (D) Quantification of endothelial nuclei per focal plane in phenotypic wild-type (n = 31) and NICD-overexpressing (n = 28) embryos. Embryos treated with DAPT contained 6.66 (s = 2.42) endothelial nuclei per section, whereas those treated with DMSO had 4.03 (s = 1.27) endothelial nuclei (ANOVA, p < 10-6). Conversely, embryos overexpressing the NICD contained 3.14 (s = 0.80) endothelial nuclei, in comparison to 4.42 (s = 1.09) nuclei per section in phenotypic wild-type siblings (ANOVA, p < 10-5), suggesting that Notch signaling negatively regulates endothelial cell number. EXPRESSION / LABELING:
|
|
Notch Signaling Positively Regulates the Number of Hematopoietic Cells during Zebrafish Development (A) Whole-mount RNA in situ hybridization showing the expression of gata1 in 18 hpf DMSO-treated or DAPT-treated embryos. Two additional markers, krox20 and otx1, which label the hindbrain and neuroectoderm, respectively, were used as positive controls. Notice the reduced expression of gata1 in DAPT-treated embryos (black arrow). (B) Transverse sections of 18 hpf DMSO- or DAPT-treated embryos visualized for TOPRO (white), gata1:GFP (green), and phalloidin (red). (C) Quantification of the hematopoietic nuclei per focal plane in DMSO-treated (n = 27) or DAPT-treated (n = 19) embryos. (D) Transverse sections of 18 hpf phenotypic wild-type siblings and Tg(hsp70l:Gal4)kca4;Tg(UAS:myc-NICD)kca3 embryos visualized for TOPRO (white), gata1:GFP (green), and c-Myc as a surrogate measure for NICD expression (red). (E) Quantification of the hematopoietic nuclei per focal plane in phenotypic wild-type siblings (n = 28) and NICD-overexpressing embryos (n = 23). DAPT-treated embryos contained 4.89 (s = 2.15) erythrocyte nuclei, in comparison to 8.07 (s = 2.38) nuclei in DMSO-treated embryos (ANOVA, p < 10-4). Conversely, embryos overexpressing the NICD contained 9.89 (s = 2.53) erythrocyte nuclei, in comparison to 6.72 (s = 1.43) nuclei per section in phenotypic wild-type siblings (ANOVA, p < 10-6), indicating that Notch signaling positively modulates the number of hematopoietic cells. EXPRESSION / LABELING:
|
|
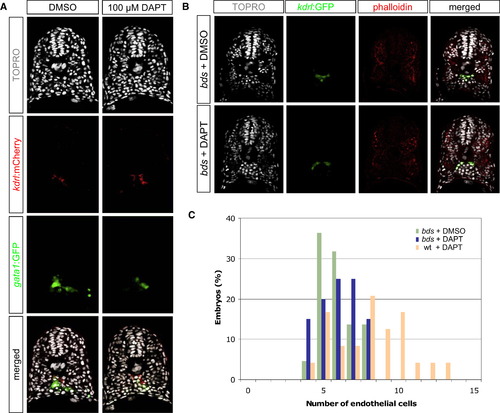
Reduced Notch Activity Causes an Increase of Endothelial Cells at the Expense of Hematopoietic Cells (A) Transverse sections of 18 hpf DMSO- or DAPT-treated embryos, visualized for TOPRO (white), kdrl:mCherry (red), and gata1:GFP (green). DAPT-treated embryos exhibited a concomitant gain of endothelial cells and loss of hematopoietic cells. (B) Transverse sections of 20 hpf DMSO- or DAPT-treated phenotypic bloodless (bds) mutant embryos, visualized for TOPRO (white), kdrl:GFP (green), and phalloidin (red). (C) Quantification of the number of endothelial nuclei per focal plane in DMSO-treated bds (n = 22), DAPT-treated bds (n = 20), and DAPT-treated wild-type sibling (n = 24) embryos. DAPT treatment in embryos lacking primitive hematopoietic cells (bds + DAPT) did not result in a change in the number of endothelial cells, suggesting that the ectopic endothelial cells observed in DAPT-treated wild-type embryos might have originated from cells that normally produce hematopoietic cells. EXPRESSION / LABELING:
|
|
Notch Signaling Functions as a Cell-Fate Switch between the Endothelial and the Hematopoietic Lineages in the Nascent Mesoderm (A) Experimental scheme of the lineage-tracing experiment. Single-cell-stage embryos were injected with DMNB-caged fluorescein-conjugated dextran. At shield stage, ten cells within the ventral margin, which predominantly gives rise to hematopoietic cells, were uncaged with UV irradiation. Subsequently, embryos were treated with either DMSO or DAPT until 18 hpf. (B) Transverse sections of 18 hpf embryos treated with DMSO or DAPT after uncaging, visualized for TOPRO (white), kdrl:GFP (green), and uncaged fluorescein (red). White arrowheads point to endothelial cells that contain uncaged fluorescein in DAPT-treated embryos. (C) Quantification of the number of endothelial nuclei with uncaged fluorescein per focal plane in embryos treated with DMSO (n = 18) or DAPT (n = 13). Embryos treated with DAPT contained 1.61 (s = 1.01) fluorescein-positive endothelial cells, in comparison to 0.16 (s = 0.37) in DMSO-treated embryos. (D) Our proposed model: Notch signaling negatively regulates endothelial cell number by limiting the mesodermal cells that can differentiate into endothelial progenitors. Attenuated Notch activity causes mesodermal cells with hematopoietic potential to differentiate into endothelial progenitors, thus increasing the number of endothelial cells. |
|
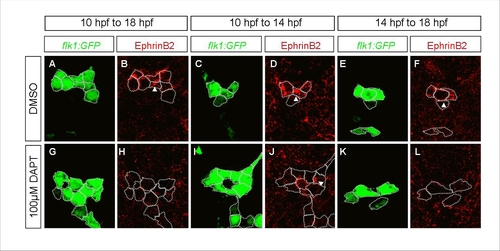
DAPT Treatment Leads to an Increased Number of Endothelial Cells at the Fifth Somite Level |
|
mibta52b Mutant Embryos Display an Increased Number of Endothelial Cells |
|
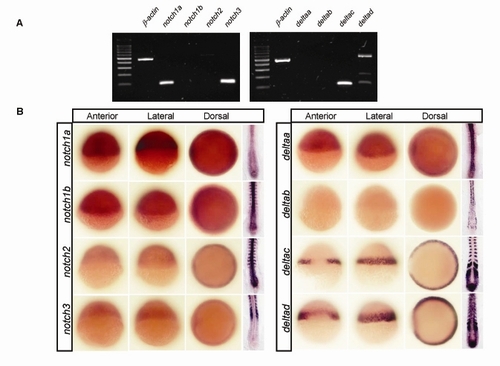
Multiple notch and delta Genes Are Expressed in the Zebrafish Gastrula |
|
A Distinct Role for Notch Signaling in the Formation of Endothelial Progenitors |
|
Cell Proliferation Is Unaffected in Embryos with Reduced Notch Signaling |
|
Notch Signaling Does Not Affect the Number of Other Nonaxial Mesoderm-Derived Lineages |
|
Gross Developmental Process Is Unaffected in Embryos with Modulated Notch Signaling |