- Title
-
Distinct populations of quiescent and proliferative pancreatic β-cells identified by HOTcre mediated labeling
- Authors
- Hesselson, D., Anderson, R.M., Beinat, M., and Stainier, D.Y.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
The principal islet contains label-retaining endocrine cells. Embryos were labeled by injection of H2B-RFP mRNA at the one-cell stage. (A and B) Confocal sections through the endoderm at 24 hpf, and (C and D) confocal projections of endodermal tissue at 52 hpf, anterior to the top, dorsal to the left; L, liver, sb, swim bladder, dp, dorsal pancreas, vp, ventral pancreas. Dotted lines delineate Tg(XlEef1a1:GFP)s854 expressing endodermal tissue (A and B) or the ventral pancreatic bud (C and D). (E–H) Confocal sections through the principal islet. (A and B) All cell nuclei are initially labeled with H2B-RFP, including the GFP positive endodermal tissue (dotted outline). (C and D) At 52 hpf, the H2B-RFP label is retained in a dorsal cluster of endodermal cells, some of which coexpress Insulin (blue). Ventral pancreatic tissue (dotted outline) has diluted the H2B-RFP label. (E) All label-retaining endodermal cells coexpress the transcription factor Islet-1, arrowhead. (F–H) A subset of label-retaining endodermal cells express Insulin (F) Glucagon (G) or Somatostatin (H), arrowheads. (E–H) All islets at this stage also contain cells that are H2B-RFP negative but Islet-1 and hormone positive, arrows. |
|
Label-retaining β-cells are derived from the dorsal pancreatic bud. Embryos were injected at the one-cell stage with H2B-RFP mRNA (A, A′, and G) or H2B-RFP mRNA and prkci MO (B, B′, C, and D). (A and B) Confocal projections of 52 hpf Tg(ptf1a:GFP)jh1 pancreata and (A2 and B′) confocal sections from each projection. (C and D) Confocal sections through the principal islet at 52 hpf. (E) Confocal projection of an islet coexpressing Tg(-4.0ins:GFP)zf5 and Tg(insulin:dsRed)m1018 at 46 hpf. (F) Quantification of Tg(ins:dsRed)m1018 expressing, and total β-cells per embryo from 24 to 46 hpf, expressed as mean ± SEM (See Table S1). (G) Confocal section through a Tg(-4.0ins:GFP)zf5 positive islet. (H) Quantification of H2B-RFP label-retaining, and total β-cells per embryo from 52 hpf to 12 dpf, expressed as mean ± SEM (see Table S1). (A and A′) β-cells that do not retain the H2B:RFP label appear by 52 hpf. (B and B2) Insulin expression is restricted to H2B-RFP positive cells in prkci morphants. (B) None of the label-retaining β-cells colocalized with Tg(ptf1a:GFP)jh1 expression, indicating that these cells derive from the dorsal bud. Some label-retaining cells do not express Insulin (arrow) and are likely other endocrine cell types. (C and D) All Islet-1 positive endocrine cells retained the H2B-RFP label when bud fusion was inhibited. (E) Recently differentiated β-cells only express GFP (green, arrow). (F) β-cells that differentiated by 28 hpf (green bars) coexpress dsRed at 46 hpf (yellow bars). (G) At 120 hpf, some β-cells (yellow, arrowhead) retain the H2B-RFP label while others (green, arrow) do not. (H) Yellow bars: The number of H2B-RFP label-retaining β-cells does not change from 52 hpf to 12 dpf. Green bars: The total number of β-cells increases over this period. |
|
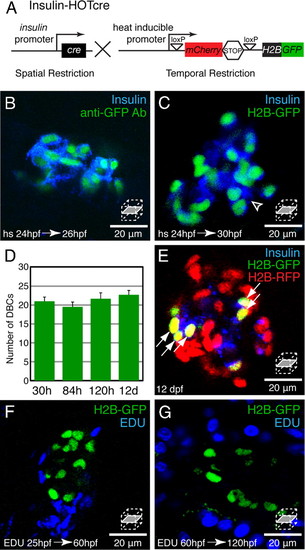
Dorsal bud derived β-cells (DBCs) are quiescent. (A) Insulin-HOTcre, Heat-inducible Overexpression of Transgenes in an insulin: Cre restricted domain. Expression of Cre in β-cells excises the mCherrySTOP cassette and permits heat-inducible expression of H2B-GFP. (B–G) Insulin-HOTcre embryos were heat-shocked at 24 hpf. (B and C) Confocal sections through an islet stained for Insulin and GFP at 26 hpf (B) or Insulin at 30 hpf (C). (D) Quantification of H2B-GFP positive cells per embryo after 24 hpf heat-shock from 30 hpf to 12 dpf, mean ± SEM (Table S1). (E) Insulin-HOTcre embryos were injected with H2B-RFP mRNA at the one-cell stage, and heat-shocked at 24 hpf. Confocal section of the principal islet stained for Insulin at 12 dpf. The mCherry heat-shock induction marker is degraded by 12 dpf (See Fig. S2A). (F and G) Embryos were labeled by pericardial EDU injection at 25 (F) or 60 (G) hpf. Confocal sections through islets at 60 (F) or 120 (G) hpf. (B) All Insulin-positive cells coexpress H2B-GFP at 26 hpf. (C) β-cells that differentiated after the heat-shock at 24 hpf express Insulin but do not express H2B-GFP (arrowhead). (D and E) The number of H2B-GFP positive cells after a 24 hpf heat-shock does not significantly change during development (D). Furthermore, all H2B-GFP-positive cells labeled after heat-shock at 24 hpf, also retain the H2B-RFP label (arrows, E). (F and G) Many cells within the pancreas divide during embryonic and larval development (blue nuclei). However, β-cells labeled by Insulin-HOTcre induction at 24 hpf did not exhibit EDU incorporation (n = 5 islets for each condition). |
|
The population of ventral bud derived β-cells (VBCs) expands by proliferation and neogenesis. (A and B) Insulin-HOTcre embryos were injected with H2B-RFP mRNA at the one-cell stage and heat-shocked at 120 hpf. (A) Confocal section of an islet at 126 hpf. (B) Quantification of VBCs per embryo at 126 hpf and 12 dpf, mean ± SEM (Table S1). (C and D) Insulin-HOTcre; Tg(ins:CFP-NTR)s892 embryos heat-shocked at 120 hpf. (C) Confocal section of an islet at 12 dpf. (D) Quantification of β-cells per embryo that had differentiated before the heat-shock (green bars) and those that differentiated after the heat-shock (blue bars), mean ± SEM (Table S1). (A and B). Some cells (DBCs) express both H2B-RFP and H2B-GFP (arrowhead); other cells (VBCs) express only the H2B-GFP label (arrow). The number of VBCs increased between 126 hpf and 12 dpf, reflecting division of existing VBCs. (C and D) β-cells marked by Insulin-HOTcre labeling at 120 hpf express H2B-GFP and CFP (arrow), whereas β-cells that differentiated after the heat-shock only express CFP (arrowhead). β-cell neogenesis contributes a significant proportion of β-cells during larval development (blue bars). (E) β-cells include both DBCs (yellow) and VBCs (green and blue) and the population of VBCs expands through a combination of proliferation (green) and neogenesis from the extra-pancreatic duct (blue), and later, intrapancreatic ducts (data not shown). |
|
H2B-RFP is retained in dorsal bud derived 7beta;-cells during embryonic and larval development. All embryos were injected with H2B-RFP mRNA at the one cell stage. (A) Confocal sections of prkci |
|
HOTcre controls. (A and B) Confocal sections of islets stained with TOPRO (A) to mark nuclei. (A) The mCherry induction reporter was induced ubiquitously and H2B-GFP was expressed specifically in β-cells by heat-shock of Insulin-HOTcre embryos at 24 hpf. By 84 hpf, mCherry is expressed at low levels in many tissues. Within the slowly dividing endocrine compartment, mCherry is initially retained as bright puncta. The intensity of the mCherry signal is reduced by 120 hpf and reaches the limit of detection by 12 dpf. Without heat-shock treatment, H2B-GFP is not detected in the islet. In the absence of Tg(ins:Cre; cryaa:Venus)s924, H2B-GFP is not detected in the islet following heat-shock treatment at 24 hpf. (B) H2B-GFP was expressed specifically in β-cells by heat-shock of Insulin-HOTcre embryos at 120 hpf. By 126 hpf, H2B-GFP is expressed in all Insulin positive cells. |