- Title
-
Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta
- Authors
- Wilkinson, R.N., Pouget, C., Gering, M., Russell, A.J., Davies, S.G., Kimelman, D., and Patient, R.
- Source
- Full text @ Dev. Cell
|
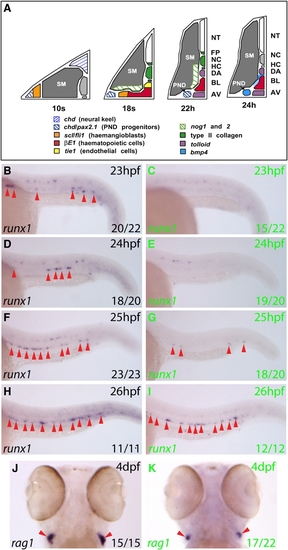
Ectopic Hedgehog Signaling Expands tbx20 Expression into the Ventral DA but Does Not Suppress the Initiation of HSC Gene Expression (A) Diagram of transverse section illustrating DA polarization in the zebrafish trunk at 24 hpf. (B–G) Embryos treated with DMSO or PMA from 15S and probed for tbx20 or runx1 at 24 hpf. Dorsal and ventral DA expression (yellow and red arrows) are shown. PMA treatment expands tbx20 expression into the ventral DA ([C] and [E] compared to [B] and [D]), but runx1 is unaffected (F and G). AV, axial vein; DA, dorsal aorta; NC, notochord; NT, neural tube; PMA, purmorphamine; S, somite. |
|
Bmp Signaling Is Required for Initiation of the HSC Program in the Ventral DA (A) Gene expression summary as progenitors migrate to the midline and form blood and DA/AV endothelium. The DA region becomes conducive to Bmp signaling by 24 hpf. (B–I) Expression of runx1 (arrows) in embryos heat shocked at 21 hpf. runx1 expression in the DA at 23–26 hpf (GFP-, black letters) was substantially reduced in GFP+ embryos (green letters) at 23–25 hpf. Representative embryos are depicted, and numbers with or without runx1 staining were scored. (J and K) rag1 expression in thymi at 4 dpf heat shocked at 21 hpf. 17/22 GFP+ embryos had reduced rag1 expression compared to GFP- embryos, in which rag1 staining showed little variation. The remaining 5/22 GFP+ embryos exhibited wild-type rag1 expression. AV, axial vein; BL, primitive blood; DA, dorsal aorta; HC, hypochord; NC, notochord; NT, neural tube; PND, pronephric duct; SM, somitic mesoderm. |
|
Bmp Signaling Is Required for Maintenance of runx1 Expression in the Ventral DA but Not for Maintenance of the Arterial Program (A–H) runx1 expression (arrows) in embryos heat shocked at 22 hpf. runx1 initiated normally, was lost by 24 hpf in GFP+ embryos (green letters) compared to GFP- embryos (black letters), but recovered by 26 hpf. Representative embryos are shown. Embryos containing a few runx1+ cells were scored as negative. (I–P) deltaC expression in the DA (arrows) in embryos heat shocked at 21 hpf was unaffected by inhibition of Bmp signaling. (Q and R) notch5 expression in the ventral wall of the DA (arrow) in sectioned embryos heat shocked at 21 hpf was normal. |
|
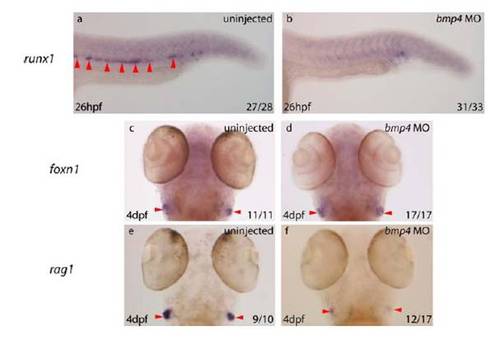
bmp4 Is Required for HSC Emergence within the Ventral DA (A–D) runx1 expression was absent from the DA (arrows) in 8/12 bmp4 morphants at 28 hpf (C and D), while the remaining 4/12 exhibited reduced expression compared with uninjected controls (A and B). (E–H) At 28 hpf, 17/21 bmp4 morphants exhibited absent or substantially downregulated cmyb expression in the DA (red arrows). (I and J) At 4 dpf, 27/34 bmp4 morphants exhibited substantial downregulation of ikaros expression within the developing thymi (arrows). The remaining 7/34 exhibited wild-type expression. (K and L) At 4 dpf, 26/32 bmp4 morphants exhibited substantially reduced expression of rag1 in developing thymi (arrows), of which 7 exhibited no rag1 expression. The remaining 6/32 were wild-type. (M and N) Dorsolateral views of the developing pronephros at 5 dpf depicting substantial downregulation of cmyb expression (outlined) in 22/29 embryos, of which 4/29 exhibited no expression. The remaining 7/29 were wild-type. (O) Diagram of zebrafish trunk depicting polarization of the DA by dorsal Hedgehog and ventral Bmp signaling, mirroring the neural tube. |
|
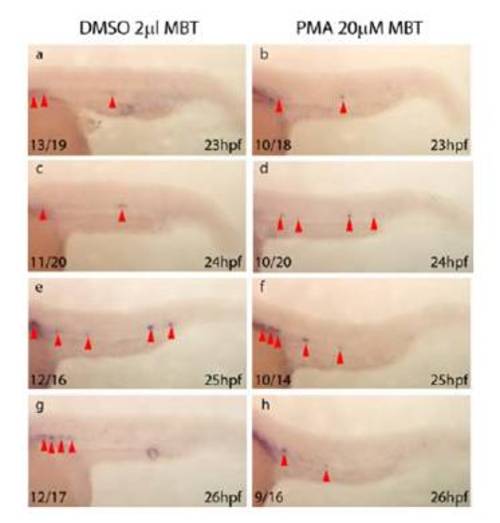
Purmorphamine is a Small Molecule Agonist of Hedgehog Signalling in Zebrafish Embryos a-c) Phenotype of 26hpf control embryos treated from before midblastula transition (MBT) with 2μl DMSO. d-f) Phenotype of 26hpf embryos treated prior to MBT with 20μM purmorphamine displaying an altered somitic chevron angle (black arrowheads) and neural defects characteristic of hedgehog activation (blue arrowheads). Pooling of primitive blood is also evident (red arrowhead and asterisk), arising from absence of circulation due to loss of the axial vein (data not shown). g) Anterior view of 24hpf embryo treated with 2μl DMSO from before MBT (n=20/20) illustrating normal expression of ptc1 (green arrowheads). h) Anterior view of 5s control embryo treated with 2μl DMSO prior to MBT (n=19/20) illustrating normal expression of pax2.1 (green arrowhead) within the forebrain. i) 26hpf embryo treated with 2μl DMSO from before MBT (n=20/20) illustrating normal expression of pax6a within the forebrain (green arrowhead). j) Anterior view of 24hpf embryo treated with 20μM purmorphamine from before MBT (n=17/20) illustrating increased ptc1 expression (green arrowheads). k) Anterior view of 5s embryo treated with 20μM purmorphamine from before MBT (n=14/20) illustrating increased pax2.1 expression within the forebrain (green arrowhead) l) 26hpf embryo treated with 20μM purmorphamine from before MBT (n=16/20) illustrating a reduced area of expression of pax6a within the forebrain (green arrowhead). |
|
Purmorphamine Treatment Does Not Affect Initiation or Maintenance of gfi1 Expression Within the Zebrafish DA The initiation of expression of an additional marker of HSCs (Hock et al., 2004) (Zeng et al., 2004), gfi1 (Dufourcq et al., 2004), is unaffected following elevation of Hh. It is of note that, on average, 66% of wild-type zebrafish embryos express gfi1 within the DA at any time between 23 and 30hpf, which suggests that its expression within the HSCs is cycling (data not shown). a-h) Initiation and maintenance of gfi1 in the DA (red arrowheads) is unaffected in embryos treated with purmorphamine from mid blastula transition (b, d, f, h) in comparison to DMSO treated controls (a, c, e, g). Since a percentage of embryos fail to express gfi1 at each time point (data not shown), indicating that gfi1 expression is highly dynamic and may be cycling within the DA, embryo numbers relate to the number of embryos expressing gfi1 within the DA and not the number of positive cells. |
|
bmp4 Transcripts are Expressed Throughout the Zebrafish Trunk, Found Polarised Under the Ventral Wall of the Dorsal Aorta During the Initiation of Definitive Haematopoiesis and Chordin Transcripts are Present Within the Pronephric Duct Progenitors and the Pronephric Duct. a) Whole-mount in situ hybridisation with riboprobe to bmp4 in 23hpf wild-type embryo. bmp4 transcripts are present in the pronephric duct (PND) in the anterior trunk (blue arrowhead) and in the ventral mesenchyme under the DA (red arrowhead) in the posterior trunk. 10μm thick sections are shown in panels b, c and e. b) Transverse section through 24hpf embryo subjected to in situ hybridisation for bmp4 illustrating polarisation of bmp4 transcripts within the ventral mesenchyme under the DA (red arrowheads). c) Transverse section of 22hpf embryo stained for chordin (chd). Expression of chd within the pronephric ducts is illustrated (blue arrowheads). d) Flat mounted 5s embryo stained for pax2.1 and chd. Co-expression of pax2.1 and chd is illustrated within pronephric duct progenitors (blue arrowheads). e) Transverse section of 10s embryo subjected stained for chd. Expression of chd within the pronephric duct progenitors is illustrated (blue arrowheads). f) Flat mounted 10s embryo stained for scl and chd. chd is found lateral to scl within pronephric duct progenitors (blue arrows). No co-expression of chd and scl was detected. AV, axial vein; DA, dorsal aorta; NT, neural tube; NC, notochord; PND, pronephric duct; S, somite; VM, ventral mesenchyme. |
|
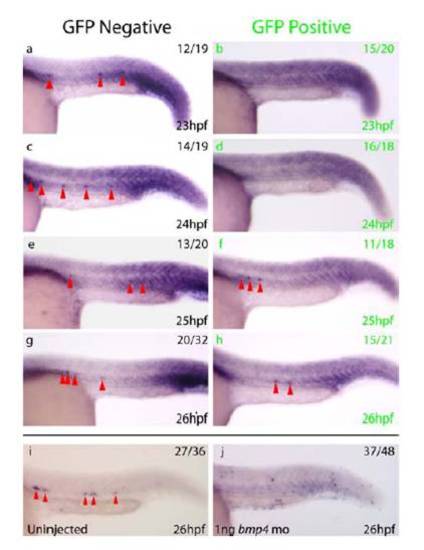
Heat Shock Induced Expression of tBR Leads to Reduced Levels of Phosphorylated Bmp Receptor Smads Whole-mount immunohistochemistry for phosphorylated Smad1, 5, 8 in 23-26hpf embryos from a tBR heterozygous outcross subjected to a 21hpf heat shock. a, c, e, g) Nuclear staining is clearly visible within the developing myotome of GFP negative embryos (black letters). b, d, f, h) Nuclear staining is substantially reduced in GFP positive embryos (green letters) at 23hpf, 24hpf and 25hpf in comparison to GFP negative embryos at the same stage. By 26hpf, differences in nuclear staining between GFP positive and GFP negative embryos are much less, indicating turnover of dominant negative receptor and recovery of Bmp signalling within the trunk to wild-type levels at this time. |
|
Heat Shock Induced Expression of tBR Leads to a Loss of runx1 Expression Within the DA Without Affecting Neural runx1 Expression. a-h) Expression of runx1 within the DA (red arrowheads), olfactory placode (blue arrowheads) Rohon-Beard sensory neurons (yellow arrowheads) and otic vesicle (black arrowheads) in embryos heatshocked at 21hpf. Whereas runx1 is expressed in the dorsal aorta, olfactory placode, otic vesicle and Rohon-Beard sensory neurons at 23hpf and 24hpf in control GFP- embryos (black letters), it is specifically lost from the dorsal aorta in GFP+ embryos (green embryos), whilst retaining its neural expression. |
|
Expression of gfi1 Within the DA is Lost in a Similar Manner to runx1 Following Bmp Inhibition a-h) Expression of gfi1 (red arrowheads) in embryos heat shocked at 21hpf. Whereas gfi1 is expressed from 23-26hpf in two thirds of control GFP- (black letters) embryos, gfi1 is lost from the DA in 75% of GFP+ (green letters) embryos at 23hpf and 89% of GFP+ embryos at 24hpf, in a similar pattern to that observed for runx1 following induction of tBR at the same stages. i, j) Whilst gfi1 is expressed within the DA at 26hpf (red arrowheads) in 75% of uninjected controls (i), expression of gfi1 is absent from the DA in 77% of embryos injected with bmp4 morpholino (j). Taken together, these results indicate that temporal inhibition of Bmp signalling (a-h) results in temporal inhibition of gfi1 followed by recovery, but that permanent inhibition of Bmp via bmp4 morpholino injection results in a more permanent inhibition of gfi1 expression within the DA in a similar manner to that observed for runx1. Embryos were over stained to ensure that lack of gfi1 expression in the DA was not an artefact of reduced staining. |
|
Initiation and Maintenance of HSC Gene Expression are Unaffected in Dino Mutants a-f) Whereas initiation (a, b, d, e) and maintenance (c, f) of runx1 expression within the DA of dino mutants, chdtt250 (Schulte-Merker et al., 1997), was not significantly affected in comparison to wild type embryos from the same in cross (red arrowheads), the expression of runx1 within the posterior ICM was substantially increased in dino mutants (blue arrows). g-j) gfi1 expression within the DA of dino mutants behaves in a similar manner to runx1 in that it also exhibits no significant differences from wild type expression (red arrowheads), but is ectopically expressed within the posterior ICM (blue arrowheads). k-l) Maintenance of arterial tbx20 is unaffected in the DA of dino mutants in comparison to wild type embryos from the same in cross (yellow arrowheads). The Bmp-dependent expression of tbx20 within the ventral cells under the yolk cell extension (Supp Fig 5, green arrowheads) is substantially increased in dino mutants compared to wild type embryos (orange arrowheads). |
|
Induction of tBR Line by Heat Shock at 21hpf Does Not Affect The Polarisation of tbx20 to the Dorsal Wall of the DA. a-h) Expression of tbx20 (red and green arrowheads) from 23-26hpf in embryos heat shocked at 21hpf. Whereas tbx20 expression within the dorsal aorta (green arrowheads) is unaffected in GFP- (black letters) or GFP+ embryos (green letters), GFP+ embryos demonstrate a loss of ventral expression under the yolk cell extension (red arrowheads), as observed previously (Pyati et al., 2006). i, j) Transgenic embryos heat shocked at 21hpf were subjected to in situ hybridisation for tbx20, sectioned to a thickness of 10μm and counterstained with nuclear fast red. Exclusion of tbx20 from the ventral wall of the DA was observed in both GFP- (i) and GFP+ embryos (j) at 24hpf (green arrows). Abbreviations: NC, notochord. |
|
The Arterial ProGramme is Unaffected by Targeted Knockdown of bmp4 a, b) gata1 expression in uninjected 10 somite stage embryos was normal within the posterior lateral mesoderm (PLM) (a), whereas bmp4 morphants demonstrate ectopic gata1 expression in the PLM, posterior to the tailbud (b) (red arrowhead). c-f) Expression of tbx20 within the DA was unaffected in control embryos or bmp4 morphants (red arrowhead). Ventral expression of tbx20 under the yolk cell extension was not lost in bmp4 morphants, presumably because other Bmp family members are important in its maintenance. g-j) Pan-arterial expression of deltaC was unaffected in control embryos or bmp4 morphants (red arrowhead). |
|
Targeted Knockdown of bmp4 Results in a Loss of HSC-Derived Thymocytes All embryos depicted are from the same experiment, representative embryos are shown. |
Reprinted from Developmental Cell, 16(6), Wilkinson, R.N., Pouget, C., Gering, M., Russell, A.J., Davies, S.G., Kimelman, D., and Patient, R., Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta, 909-916, Copyright (2009) with permission from Elsevier. Full text @ Dev. Cell