- Title
-
Olfactory neural circuitry for attraction to amino acids revealed by transposon-mediated gene trap approach in zebrafish
- Authors
- Koide, T., Miyasaka, N., Morimoto, K., Asakawa, K., Urasaki, A., Kawakami, K., and Yoshihara, Y.
- Source
- Full text @ Proc. Natl. Acad. Sci. USA
|
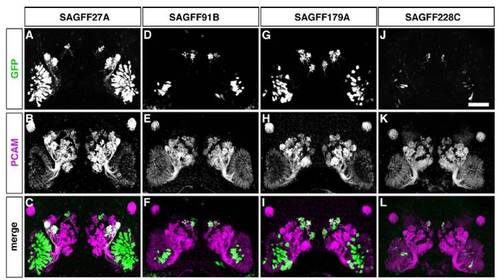
Gene trap zebrafish lines with Gal4FF expression in OSN subsets innervating distinct glomeruli. GFP expression patterns were examined in 5-day-old larvae of the 4 gene trap Gal4FF-expressing lines (A1–A7, SAGFF27A; B1–B7, SAGFF91B; C1–C7, SAGFF179A; and D1–D7, SAGFF228C) crossed with the UAS:GFP reporter strain. (A1–D1) Lateral views of transgenic larval heads with GAL4FF-driven GFP fluorescence in the OE (arrowheads). In SAGFF91B and SAGFF179A, additional fluorescent signals are observed. (B1) The otic vesicle (OV), pineal (P), and spinal cord (SC) in SAGFF91B. (C1) The ventral mandibular arch (VMA) and SC in SAGFF179A. (A2–D2) Frontal views of larvae showing GFP fluorescence in subsets of OSNs innervating the OB (dashed circles) with different patterns. (A3–D6) Whole-mount double-immunofluorescence labeling with antibodies against GFP (A3–D6; green) and PCAM (A3–D3; magenta), Gαolf/s (A4–D4; magenta), calretinin (A5–D5; magenta), or SV2 (A6–D6; magenta). (A7–D7) Schematic diagrams illustrating different patterns of glomerular innervation by Gal4FF-expressing OSNs in the gene trap lines. The 4 lines show different combinations of glomerular targeting in the medial cluster (mG1–6, blue). SAGFF27A and SAGFF228C also show strong and weak GFP expression, respectively, in OSNs innervating the lateral glomeruli (lG, red). avG, anteroventral glomeruli; dG, dorsal glomeruli; mG, medial glomeruli; pG, posterior glomeruli. [Scale bars: (in D1), A1–D1, 200 μm; (in D2), A2–D6, 50 μm.] EXPRESSION / LABELING:
PHENOTYPE:
|
|
Conserved patterns of differential glomerular innervation of GFP-positive olfactory axons in 3 gene trap lines in adult zebrafish: (A1–A9) SAGFF27A, (B1–B9) SAGFF91B, and (C1–C9) SAGFF179A. (A1–C3) Whole-mount OBs with GFP fluorescence viewed from dorsal (A1–C1), ventral (A2–C2), and lateral (A3–C3) sides. (A4–C8) Horizontal sections of OBs triple-labeled with antibodies against GFP (green), PCAM (red), and SV2 (blue). For individual lines, 5 sections are shown from the dorsal-most (A4–C4) to ventral-most (A8–C8) levels. (A9–C9) Schematic diagrams illustrating different patterns of glomerular innervation by GAL4FF-expressing OSNs in the adult gene trap lines. In SAGFF27A, GFP-positive olfactory axons innervate the lateral chain (lc, red), the ventral glomeruli (vG, pink), and 1 of the mediodorsal posterior glomeruli (mdpG2, blue). In SAGFF91B, GFP-positive olfactory axons innervate 2 of the mediodorsal posterior glomeruli (mdpG2 and mdpG3, blue). In SAGFF179A, GFP-positive olfactory axons innervate the mdpG1-3 (blue), the mediodorsal glomerulus (mdG, blue), and the ventroposterior glomerulus (vpG, light blue). (Scale bars: 100 μm.) EXPRESSION / LABELING:
|
|
Various patterns of GFP expression in the gene trap (A–I) and enhancer trap (J–P) zebrafish lines. Frontal views of 5-day-old larvae with ventral to the bottom. SAGFF27A (A), SAGFF91B (B), SAGFF179A (C), and SAGFF228C (D): distinct subsets of OSNs (see Results for details). (E) SAGFF187A: nonneuronal cells in the OE and neuromasts. (F) SAGFF212C: a subset of OSNs and cells in the forebrain. SAGFF70A (G), SAGFF120A (H), and SAGFF36A (I): cells in forebrain and small subsets of OSNs. (J) hspGFF3A: a subset of OSNs. (K) hspGFF3B: OSNs and epidermis. hspGFF32A (L), hspGFFDMCIA (M), and hspGGFF10B (N): OSNs and forebrain neurons. hspGGFF20A (O) and hspGGFF25A (P): nonneuronal cells in the OE and cells in the forebrain. (Q) A schematic diagram illustrating the frontal view of zebrafish larva. FB, forebrain; OB, olfactory bulb; OE, olfactory epithelium. (Scale bar: 50 μm.) |
|
PCAM expression in all the OSN axons. GFP expression patterns were examined in 5-day-old larvae of the 4 gene trap Gal4FF-expressing lines (A–C, SAGFF27A; D–F, SAGFF91B; G–I, SAGFF179A; and J–L, SAGFF228C) crossed with the UAS:GFP reporter strain. Whole-mount double-immunofluorescence labeling with antibodies against GFP (A, D, G, and J; green in C, F, I, and L) and PCAM (B, E, H, and K; magenta in C, F, I, and L). Single-label gray-scale images of PCAM demonstrate that PCAM is expressed in all the OSN axons, although its expression level is not equal among the axons. In particular, the axons projecting to the medial glomerular cluster show a relatively weak expression of PCAM compared with other axons. (Scale bar: 50 μm.) |
|
Differential GFP expression in distinct types of OSNs in 2 transgenic fish lines, SAGFF27A (A–D) and OMP:Gal4FF (E–H). Adult OE sections were triple-labeled with antibodies against GFP (A and E; green in D and H), calretinin (B and F; red in D and H), and Gαolf/s (C and G; blue in D and H). In the SAGFF27A line, GFP is expressed in short dendrite-bearing and calretinin-positive OSNs in the apical layer of the OE (A, B, and D), suggesting that Gal4FF/GFP-expressing cells are the microvillous OSNs. In contrast, in the OMP:Gal4FF fish, GFP is observed in long dendrite-bearing OSNs, the cell bodies of which are located in the basal layer of the OE (E, G, and H). Arrows indicate the dendritic knobs that are positive for both GFP and Gαolf/s on the epithelial surface. These results indicate that Gal4FF/GFP-expressing cells in the OMP:Gal4FF fish are the ciliated OSNs. (Scale bar: 10 μm.) |
|
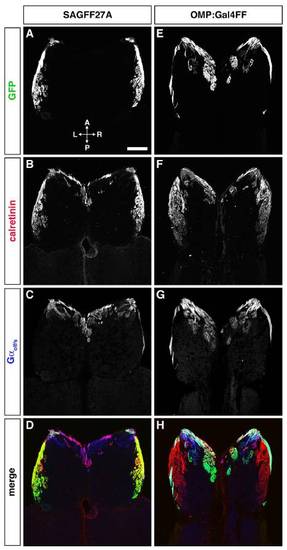
Differential glomerular innervation by distinct OSN axons in SAGFF27A (A–D) and OMP:Gal4FF (E–H). Horizontal sections through the OB were triple-labeled with anti-GFP (A and E; green in D and H), anti-calretinin (B and F; red in D and H), and anti-Gαolf/s (C and G; blue in D and H) antibodies. In the SAGFF27A line, GFP-expressing microvillous OSNs project their axons exclusively to calretinin-positive glomeruli in the lateral region of the OB. In contrast, GFP-expressing ciliated OSNs in the OMP:Gal4FF fish project their axons mainly to Gαolf/s-positive glomeruli in the anterior and medial regions of the OB. (Scale bar: 100 μm.) |