- Title
-
A novel role for MAPKAPK2 in morphogenesis during zebrafish development
- Authors
- Holloway, B.A., Gomez de la Torre Canny, S., Ye, Y., Slusarski, D.C., Freisinger, C.M., Dosch, R., Chou, M.M., Wagner, D.S., and Mullins, M.C.
- Source
- Full text @ PLoS Genet.
|
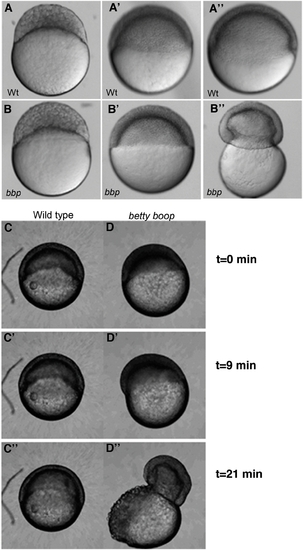
bbp embryos constrict at the margin at 50% epiboly, bursting the yolk. Still images of WT and bbp embryos at the 1000-cell stage (A, B), 50% epiboly (A′, B′), and 50% epiboly at the burst (A″, B″). bbp embryos constrict just as they reach 50% epiboly. (C and D) Selected frames from time-lapse movies of WT and bbp embryos showing that the constriction occurs rapidly once the embryos have reached 50% epiboly. PHENOTYPE:
|
|
Characterization of bbp phenotype. In situ hybridization of goosecoid (gsc) in WT (A) and bbp (D) and no tail (ntl) in WT (B) and bbp (E) embryos at 50% epiboly. Microtubule array forms normally in bbp embryo (F) at the approach to 50% epiboly as in WT (C). G, H Internalization of Rhodamine-dextran through endocytosis in the YSL at 50% epiboly in WT (G) and a bbp (H) embryo. Rhodamine-Phalloidin staining in WT (I) and bbp (J) embryos at 50% epiboly. White brackets show punctate actin band in YSL, arrowheads indicate actin at EVL margin. Hypertonic Ringer's solution extended development of constricting bbp embryos. bbp embryo ni E3 medium constricts at 50% epiboly and yolk globules (black arrowheads) burst through the yolk membrane (K). bbp embryo reared in hypertonic medium constricts, while maintaining yolk membrane integrity (L), allowing the blastoderm to heal and survive to 24 hpf (M). Mutants can undergo gastrulation movements and generate a general body plan at 24 hpf. WT sibling reared in hypertonic medium develops normally, shown at 50% epiboly (N). YSN of WT embryo labeled with Sytox Green (O–R); both I-YSN (bracket) and E-YSN (arrowhead) change little during this short time interval. (S–V) bbp I-YSN are initially distributed normally under the blastoderm, but retract rapidly to a small region of the I-YSL (arrow) when the margin constricts, just before yolk cell rupture. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Selected frames of ratiometric images of WT (A) and bbp at early epiboly (B) and bbp at 40% epiboly (C). Algorithm of the calcium release representing the frequency and location of fluxes for the duration of the time course of early WT embryo in A (D), early bbp embryo in B (E), and 40% epiboly bbp embryo in C (F). Composites represent data collected over 1 hour of imaging of an individual embryo. PHENOTYPE:
|
|
Whole blastoderm transplants show yolk cell domain of function. Embryos were injected with rhodamine-dextran (bbp) or sytox green dye (WT) at the 1-cell stage. Blastoderms were separated from yolks and re-adhered creating chimeric embryos. (A) Schematic representation of the blastoderm transplant. A chimeric embryo (B–B′″) containing a WT yolk (B′) and bbp blastoderm (B″) progresses through epiboly properly (B′″, 80% epiboly). A chimeric embryo (C–C″) containing a WT blastoderm (C′) and bbp yolk (C″) constricts at 50% epiboly, showing that Bbp functions in the yolk cell during epiboly. Bright field images of chimeric embryos, B, C. WT derived tissue, B′, C′. bbp derived tissue, B″, C″. Merge of images at 80% epiboly in B′″. PHENOTYPE:
|
|
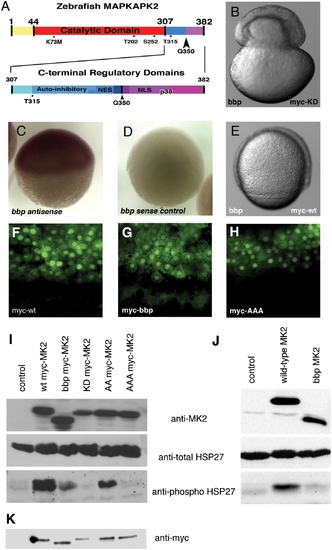
Zebrafish MAPKAPK2 protein schematic, sub-cellular localization, and kinase activity. (A) Top panel: Full length protein of 382 amino acids, with catalytic domain (red) adjacent to the carboxy-terminal regulatory domains (blue, purple). Residues T202, S252, and T315 (*) are phosphorylated by p38 MAPK. K73M is the kinase dead mutation generated. The Q350 residue (black arrowhead) is mutated to a premature stop codon in bbp, thus creating a truncated protein lacking the most carboxy-terminal regulatory domains (purple). Bottom panel: Scale representation of carboxy-terminal regulatory domains, including the auto-inhibitory helix that slightly overlaps an NES, and the NLS that also contains residues required for p38 MAPK docking (p38). (B) bbp embryo injected with kinase-dead full-length myc-tagged MAPKAPK2 (MK2), which does not rescue; shown at 50% epiboly when the constriction initiated. (C,D) Wholemount in situ hybridization of MK2 (bbp) antisense and control sense probes at 50% epiboly in WT embryos. (E) bbp embryo injected with full-length myc-tagged MK2 mRNA at the 1-cell stage is rescued; shown at 75% epiboly. (F–H) Anti-myc antibody staining of WT (bbp/+) embryos expressing WT (F), bbp (G), and a non-phosphorylatable T202A/S252A/T315A triple mutation (H) of myc-tagged MK2 (100 pg mRNA injected). I. Western blot of HeLa cell extracts expressing a vector control (lane 1), full-length myc-tagged MK2 (lane 2), truncated myc-tagged Bbp MK2 (lane 3), full-length kinase dead myc-tagged MK2 (lane 4), T202A/T315A myc-tagged MK2 (lane 5), T202A/S252A/T315A myc-tagged MK2 (lane 6). (J) Western blotting of HeLa cell extracts expressing a vector control (lane 1), full-length untagged MK2 (lane 2), or truncated untagged Bbp MK2 (lane 3). (I, J) HeLa cell extract blots were probed for total MK2 protein (MK2) (top panels), total HSP27 protein (middle panels), and phosphorylated HSP27 protein (bottom panels). (K) Western blot probed with anti-myc of embryo extracts either uninjected (lane 1) or expressing myc-tagged MK2 constructs, as described in I. |
|
Microinjection of DNp38a mRNA phenocopies bbp. WT embryos microinjected with 250 pg of DsRed2 mRNA (A) or DNp38a mRNA (B), which causes a bbp phenocopy. PHENOTYPE:
|