- Title
-
The extracellular matrix protein TGFBI promotes myofibril bundling and muscle fibre growth in the zebrafish embryo
- Authors
- Kim, H.R., and Ingham, P.W.
- Source
- Full text @ Dev. Dyn.
|
Tgfbi-GFP localises to myosepta and skeletal muscle fibres. Transient mosaic expression of a cDNA encoding a GFP-tagged form of Tgfbi downstream of the α-actin promoter. A,B: The fusion protein accumulated in a striated pattern along individual muscle fibres (shown in detail in E). In addition, protein localised along the notochord (A, arrow) and to the vertical myosepta (arrowhead) at 50 hpf. C: Transverse section of muscle fibres labelled with Lyn-tdTomato (red) reveals its localisation along the sarcolemma and also along the T-tubules that surround myofibrils. D: Perinuclear accumulation of the fusion protein is also apparent (blue: Topro3; red Phalloidin). |
|
A tgfbi splice morpholino efficiently blocks normal splicing of tgfbi mRNA. Normally spliced tgfbi mRNA in wild type embryos produced a single amplification product by RT-PCR (A, wt lane; B-a), whereas the same primers generated three different products from morphant embryo mRNA (A, MO lane). Sequencing of each band revealed that none derived from an mRNA encoding full-length Tgfbi protein. The top PCR product includes an insertion of intron between exon 4 and 5 with a stop codon in the intron (B-b). The second PCR product contains an insertion of a part of an intron, which causes the introduction of a stop codon in exon 5 (B-c). The third PCR product is produced by exon 4 skipping, which introduces a stop codon in exon 5 (B-d). |
|
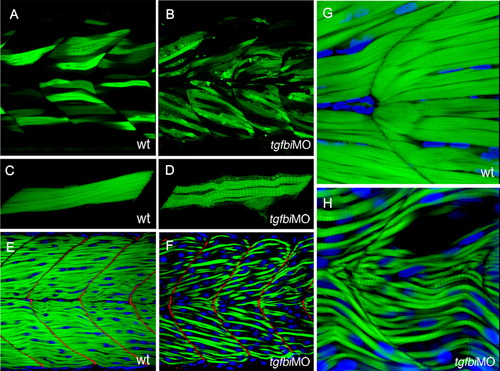
Attachment of muscle fibres to the myoseptum is not disrupted by Tgfbi knock-down. A-D: Muscle fibres in wild type and tgfbi morphant embryos that have been scatter labelled by transient expression of the α-actin:GFP transgene and imaged at 50 hpf. Like their wild-type counterparts (A), the labelled myofibres in tgfbi morphants (B) spanned entire somites with no disconnected muscle fibres. Projections of 3D images of individual labelled fibres show that the morphant muscle fibres (D) were thinner and wavier than their wild type counterparts (C). E-H: Embryos that have been stained with FITC-conjugated Phalloidin (green) to label muscle fibres and Topro3 (blue) to label nuclei. In E and F, staining for Vinculin (red) reveals that the vertical myosepta were not affected by the loss of Tgfbi. The fibres in morphant embryos (H) were much thinner and less closely packed than in wild type (G) with some empty spaces between them. |
|
Loss of Tgfbi causes lateral detachment of muscle fibres. Live images of Tg(acta1:GFP)zf13 50-hpf control and morphant embryos. A: In wild type embryos, the muscle fibres are tightly connected to each other along their length. B: In tgfbi start site morphant embryos, by contrast, empty gaps were apparent in between muscle fibres. C: The same phenotype was observed following injection of a mixture of the start and splice morpholinos, each at half the concentration of that effective for single morpholinos. PHENOTYPE:
|
|
Normal myofibrillogenesis in Tg(smyhc1:GFP)i104 transgenic embryos. A: Dorsal view of an 18 somite stage embryo showing the first appearance of striated α-Actinin (red: arrow) in slow muscle fibres (marked by GFP expression). B,C: Lateral aspect of a 20-hpf embryo: α-Actinin was organised as a clear z-band (arrowhead) in anterior somites (B), but not in more posterior somites (C, arrow). D,E: Lateral aspect of 24-hpf embryo: posterior slow fibres have clearly discernible striated thin myofibrils (D) while in more anterior somites the myofibrils have become quite thick (E, arrows). F,G: Transverse sections at successive stages reveal that in slow fibre progenitors, prior to their migration away from the notochord (indicated by the yellow disc) α-Actinin distribution is quite diffuse (F), but as migration proceeds, it becomes localised to one side of the nucleus (G, arrow) and 24 hpf reveals the fully assembled myofibrils (H). I-P: Pairs of images of lateral aspects of the surface slow muscle and the underlying fast muscle at successively later stages of development: fast muscle myotubes started to express and localise α-Actinin as a single file of myofibrils at 24 hpf, much later than in slow muscle fibres (M: arrow). Myofibrillogenesis proceeds rapidly in both slow and fast lineages between 32-50 hpf. EXPRESSION / LABELING:
|
|
Fibre growth and myofibril bundling is disrupted in tgfbi morphants. Lateral (A-H) and transverse (I-P) aspects of stage matched wild type and morphant embryos. Nuclei are stained blue (DAPI) and the cell membranes are stained green (Lyn-GFP). The red signal reveals the distribution of Z bands (α-actinin) in the lateral views and myofibrils (phallodin) in the transverse sections. In fast fibres of wild type embryos, myofibrillogenesis began along the membrane at 24 hpf (A and I), and muscle fibres continued to grow to become thick myofibres by 50 hpf (D and L). Although myofibrils start to be generated along the sarcolemma in the morphant embryos at 24 hpf (E and M), myofibrillogenesis is subsequently disrupted with myofibrils becoming detached from the sarcolemma (arrows in O and P). The progressive increase in fibre diameter seen in the wild type fast muscle fibres (I-L) is not apparent in the morphant fast muscle fibres (M-P). All images were captured and are reproduced at the same magnification. |
|
Dynamic transcription pattern of tgfbi during zebrafish embryogenesis. A,B: Expression of tgfbi initiates throughout the somites but becomes spatially restricted to the lateral and ventral regions and then to the medial part of the somites (C-F). Transcript is also detectable in the migrating neural crest cells (G-I), presumptive pharyngeal arches (the future pharyngeal arches are indicated by their numbers), and the domain where future maxilla forms (arrows) (L). It is also detectable in the pectoral fins (K-M) at various stages. A-F: Transverse section views of the somites; G-J, N-R: lateral views of head and somites; K, L: ventral views of head. All the panels are arranged anterior to the left and dorsal up. EXPRESSION / LABELING:
|
|
The number and volume of myofibrils increase more in fast than in slow fibres. A-D: In wild type fast muscle fibres, myofibrils started to be generated from the membrane and grew inwards filling the whole cytoplasm by 50 hpf. I-L: By contrast, in slow fibres, myofibrils grow at one side of the cell and show a less dramatic increase in volume. E-H: The centripetal growth of the fast muscle fibres was disturbed in the morphant embryos. All the images were captured and reproduced at the same magnification. |
|
Tgfbi knockdown has little effect on slow fibre myofibrillogenensis. Although the thin fast muscle fibres were prominent features of the morphant with occasional empty cell spaces, there was no obvious difference in the volume of slow muscle fibres (arrows) between wild type and morphant embryos. |
|
Zebrafish muscle fibres contain multiple myofibrils after two days of development. To confirm that fibres in both the anterior and posterior somites consist of multiple myofibrils, the somites close to the tail end were transversely sectioned and the number of myofibrils was analysed. A: The position of the somite is indicated by an arrow. B: Muscle fibres in the middle of the somite contained around 40 myofibrils. |
|
B,C: Muscle contraction is required for the growth of myofibrils. Immobilisation of embryos by exposure to tricaine (0.004%) between 24 to 48 hpf, results in a variable effect on muscle fibre size and morphology, reminiscent of the effects seen in tgfbi morphants. A: A control (untreated) embryo (Green: Phalloidin). |