- Title
-
Identification of common and unique modifiers of zebrafish midline bifurcation and cyclopia
- Authors
- Pei, W., and Feldman, B.
- Source
- Full text @ Dev. Biol.
|
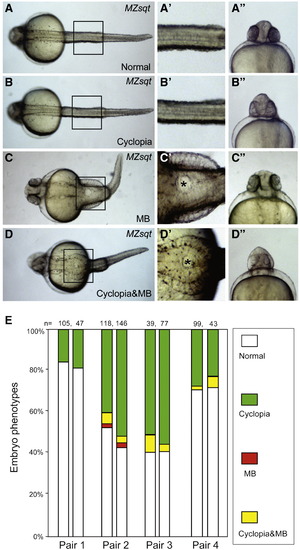
Spontaneous midline bifurcations among MZsqt embryos. (A–D) Various morphological phenotypes observed in MZsqt embryos. Four classes of embryos were observed in MZsqt embryos on 1 day post fertilization: normal-appearing embryos (A–A″), cyclopean embryos (B–B″), MB embryos (C–C″) and embryos with cyclopia and MB embryos (D–D″). The images in the central column (′) are magnifications of the boxes in the left column. The asterisks (*) label the bifurcated areas. The right hand column images (″) are frontal views of the same specimen. (E) Parental genetic background influences the incidence of MB manifestations. Repeat crosses were performed with four pairs of MZsqt parents. Embryo numbers are shown and the percentages of each embryonic phenotype indicated: normal appearing (white); cyclopia alone (green); MB alone (red) and MB + cyclopia (yellow). Pair 1, representative of the typical phenotypic spectrum, produced embryos with cyclopia but no MB. However, pairs 2 to 4 reproducibly yielded the MB phenotype. PHENOTYPE:
|
|
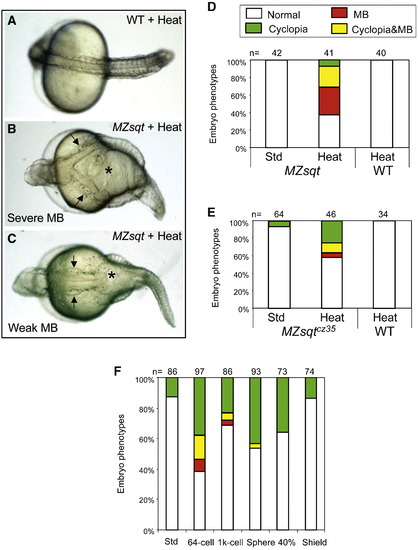
Overnight heat treatment reliably induces midline bifurcations in MZsqt embryos. (A–C) Embryonic phenotypes after overnight heat treatment. Overnight (22 h) heat treatment caused no significant defect in WT embryos (A), but led to the MB phenotype in a fraction of MZsqt embryos from every cross tested. Severe MBs extended from the trunk to the hindbrain, as demarcated by the otic vesicles (arrows) (B). Weaker MBs were limited to the trunk (C). Arrows point to otic vesicles. (D–E) Overnight heat treatment increases MB incidence in MZsqt embryos. WT and MZsqthi975/hi975 (D) or MZsqtcz35/cz35 (E) embryos were cultured under standard conditions (28 °C) or heated (34 °C) overnight starting at the 64-cell stage, then scored for the MB phenotype on 1 day post fertilization. A significant difference was observed between control and heated MZsqthi975/hi975 embryos (p = 3.00E- 08), or control and heated MZsqtcz35/cz35 embryos (p = 0.00126). (F) Early initiation of heat treatment is required to induce MB. MZsqt embryos pooled from several crosses were heated overnight, beginning at the indicated stages and scored for the MB phenotype on 1 day post-fertilization. Numbers of embryos scored are shown. Percentages for each phenotype are indicated: normal appearing (white); cyclopia alone (green); MB alone (red) and MB + cyclopia (yellow). PHENOTYPE:
|
|
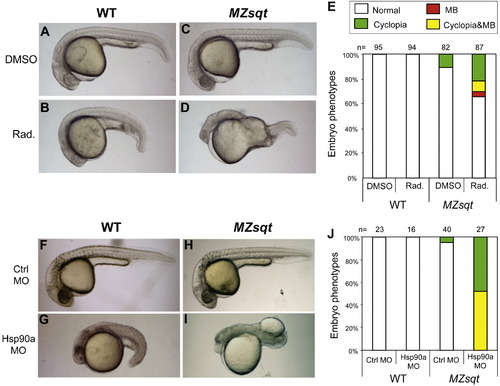
Inhibition of Hsp90 function increases the incidence of midline bifurcations. (A–D) Phenotypes of embryos injected with the Hsp90-inhibitory chemical radicicol. WT and MZsqt embryos were injected with 0.7 nl of either 27 mM radicicol in DMSO or DMSO only, and scored for the MB phenotype on 1 day post fertilization. Injection of DMSO caused no significant defects in both WT (A) and MZsqt embryos (C). Injection of radicicol in WT embryos caused slightly dark, small heads and short tails (B). Injection of radicicol in MZsqt embryos, caused these same defects, but also induced cyclopia (image not shown) and MB (D). (E) Incidence of cyclopia and MB among WT and MZsqt embryos injected with radicicol. Numbers of embryos scored are shown and correspond to a highly significant difference (p = 0.000484) in the MB rates of DMSO and radicicol treated MZsqt embryo groups. (F–I) Phenotypes of the embryos injected with an Hsp90a MO. WT and MZsqt embryos were injected with 3 ng of either control MO or an Hsp90a-targeting MO, and scored for the MB phenotype on 1 day post fertilization. The control MO caused no obvious defects in WT (F) or MZsqt embryos (H). Injection of Hsp90a MO into WT embryos generated severe defects, including dark heads and very short tails, but no MB phenotype was seen (G). Injection of the Hsp90a MO into MZsqt embryos, caused these same defects, but also induced cyclopia (image not shown) and MB (I). (J) Incidence of cyclopia and MB among WT and MZsqt embryos injected with the Hsp90a MO. Numbers of embryos scored are shown and correspond to a highly significant difference (p = 0.000001) in the MB rates of control and HSP90a MO-injected MZsqt embryos. Percentages for each phenotype are indicated: normal appearing (white); cyclopia alone (green); MB alone (red) and MB + cyclopia (yellow). PHENOTYPE:
|
|
Tissue on both sides of midline bifurcations acquires midline identity. (A) Whole-mount in situ analysis of gene expression. WT and MZsqt embryos were heat treated overnight (22 h) starting at the 64-cell stage and fixed on 1 day post fertilization for whole-mount in situ analysis. All embryos are oriented with a dorsal view, anterior on the left. (B–C) Histological sections of WT and MB embryos. WT and MZsqt embryos were heat treated for 22 h starting at the 64-cell stage and then reared for 2 more days, fixed with 4% paraformaldehyde, paraffin embedded, sectioned (5 μm/section) and stained with hematoxylin and eosin. The approximate section sites for the WT embryo (B) and the MZsqt embryo (C) are indicated. Apostrophes (') indicate the sections from the same specimen. Arrows indicate neural tubes. Arrowheads indicate guts. |
|
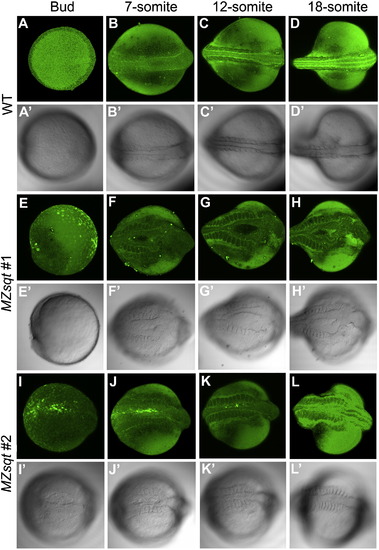
Time-lapse imaging of midline bifurcation formation and recovery. Embryos vitally-stained with fluorescent bodipy ceramide were mounted in soft agarose and incubated at 34 °C except for ∼ 15 minute interruptions at room temperature (∼22 °C) for microphotography, performed every ∼2 h. (A–D) Midline formation in heated WT embryos. Heated WT embryos developed well-defined dorsal midlines during the recording. (E–L) Midline formation in two heated MZsqt embryos. (E–H) MZsqt #1 embryos formed a large bifurcation, first visible at the 7-somite stage. (I–L) MZsqt #2 embryos displayed a small bifurcation, first visible at the bud stage (I), which recovered by the 12-somite stage (K). Because embryos were incubated and photographed at temperatures (34 °C and 22 °C) causing distinct developmental rates, the indicated stages are based on morphology, rather than time. Apostrophes (') indicate bright field images of the same specimen. Dorsal views are given throughout, with the anterior to the right. PHENOTYPE:
|
|
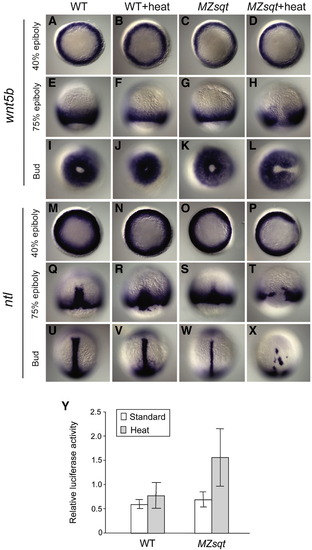
wnt5b and no tail expression prior to formation of midline bifurcations. (A–X) wnt5b (A–L) and ntl (M-X) expression during gastrulation of standard and heat-treated WT and MZsqt embryos. Embryos were cultured at 28 °C or heated (34 °C) starting at the 64-cell stage and then fixed at different stages for whole-mount in situ analysis. No difference in wnt5b expression was seen between control and heated WT embryos at all tested stages. However, heated MZsqt embryos showed reduced wnt5b expression in the dorsal region at all stages tested. Similarly, no differences in ntl expression was seen between control and heated WT embryos at all stages. However, reduced dorsal expression of ntl is obvious in control MZsqt embryos. Heated MZsqt embryos display further reduced dorsal expression of ntl at the 40% epiboly stage (P) and the 75% epiboly stage (T), and bifurcated ntl expression is often seen at the bud stage (X). Embryo orientations: 40% epiboly, animal pole view, dorsal on the right; 75% epiboly, dorsal view, animal pole on the top; bud stage, vegetal pole view, dorsal on the right for wnt5b embryos and dorsal view, animal pole on the top for ntl embryos. (Y) Up-regulated Wnt/β-catenin signaling in heated MZsqt embryos. WT and MZsqt embryos injected with 23 pg of reporter plasmids were reared at 28 °C or heated (34 °C) starting at the 64-cell stage and lysed at the bud stage for the TCF/LEF reporter assay. Average values and standard deviations from triplicate experiments are shown. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Wnt5b uniquely modifies midline bifurcation incidence; Wnt11 uniquely modifies cyclopia incidence. (A–F) Phenotypes of Wnt5b MO-injected embryos. Injection of a control MO did not cause obvious defects in either WT embryos (A), MZsqt embryos (C) or MZoep embryos (E). Injection of 6 ng of a Wnt5b MO caused shortened tails in WT (B) and MZsqt embryos (D); however, it also caused MB in MZsqt embryos (D) and MZoep embryos (F). (G) Quantification of increased MB in Wnt5b MO-injected MZsqt and MZoep embryos. Significant differences were observed in the MB rates of control MO versus Wnt5b MO-injected MZsqt embryos (p = 0.00005) and control MO versus Wnt5b MO-injected MZoep embryos (p = 0.04068), but no significant differences were seen in the cyclopia rates of control MO versus Wnt5b MO-injected MZsqt embryos (p = 0.90922), and all MZoep embryos had cyclopia. (H) Quantification of increased cyclopia in Wnt11 MO-injected MZsqt and WT embryos. Significant differences were observed in the cyclopia rate of control MO versus Wnt11 MO-injected MZsqt (p = 0.0002) and control MO versus Wnt11 MO-injected WT (p = 0.00066) embryos, but MB was not seen in any Wnt11 MO injected embryos. Numbers of embryos scored are shown and embryo percentages are indicated as follows: normal appearing (white); cyclopia alone (green); MB alone (red) and MB + cyclopia (yellow). PHENOTYPE:
|
Reprinted from Developmental Biology, 326(1), Pei, W., and Feldman, B., Identification of common and unique modifiers of zebrafish midline bifurcation and cyclopia, 201-211, Copyright (2009) with permission from Elsevier. Full text @ Dev. Biol.