- Title
-
Control over the morphology and segregation of Zebrafish germ cell granules during embryonic development
- Authors
- Strasser, M.J., Mackenzie, N.C., Dumstrei, K., Nakkrasae, L.I., Stebler, J., and Raz, E.
- Source
- Full text @ BMC Dev. Biol.
|
Expression pattern of granulito RNA and subcellular localization of the Ganulito protein. A) Whole-mount in situ hybridizations using granulito antisense RNA probe at the indicated stages. granulito is enriched at the region where the germ plasm resides (cleavage furrows, arrowheads) and is expressed in the primordial germ cells at later stages (arrowheads). B) Alignment of the zebrafish Granulito protein with those from Xenopus leavis and Homo sapiens. Red signifies conservation in all 3 species, green labels conserved substitutions. C) Subcellular localization of Granulito-EYFP. Granulito localizes specifically to germ cell granules as it colocalizes with Vasa protein. D) Germ cells of kop-granulito-dsRedex-nos1-3′UTR transgenic fish whose membrane and nucleus are labeled in green (except for the 4 hours stage where the nucleus is not labeled). Initially, germ cell granules with a large variety of sizes are observed. As development proceeds, the variation in germ cell granule size decreases. E) Granule size distribution at different stages. F) Variance of granule size at different stages. N >100 granules for each time point. |
|
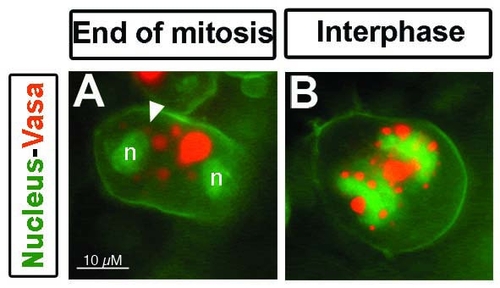
Germ cell granule behavior during cell division in live embryos. A) During interphase and before nuclear envelope breakdown (NEBD), the granules (labeled with Vasa-dsRed) show an organized localization around the nucleus (visualized with LaminB2-GFP). During early prophase, the nuclear envelop disassembles and germ cell granules change their shape (arrow). Once the envelope is completely disassembled granules that changed their shape readopt their spherical shape (arrowhead). All granules spread in the cytoplasm and are segregated into daughter cells. B) After cytokinesis, the nuclear envelope reassembles (n) and a group of granules spread in the cytoplasm readopt perinuclear localization (asterisk). Granules are labeled with Vasa-dsRed, nuclear envelope with LaminB2-GFP, and plasma membrane with farnesylated GFP. C) Movement of granules towards the nuclear envelope labeled with NUP155-GFP and visualized by confocal microscopy. Granules move directly (asterisk) to the area where the nuclear envelope is reassembling (n) during cytokinesis. All movies were recorded in 10 hpf embryos. |
|
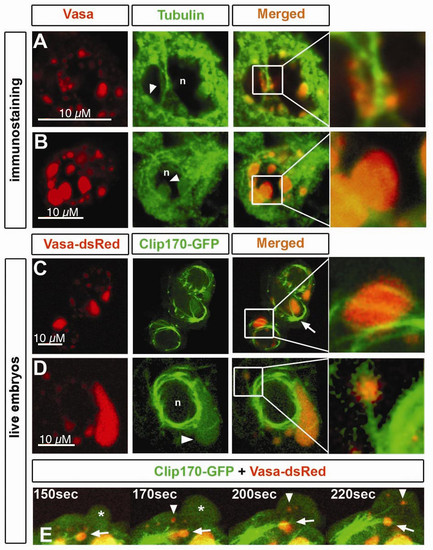
Microtubules colocalize with germ cell granules. A) Immunostaining of Vasa protein and α-Tubulin in germ cells of 6 hpf embryos. Perinuclear granules attached to microtubules are shown in detail. n and arrowhead depicts the nucleus and granules expressing Tubulin, respectively. B) At 16 hpf, germ cell perinuclear granules show expression of α-Tubulin within their structure (arrowhead). Nucleus is depicted by n. Pinhole value is 1.22. C).Germ cells in live 11 hpf embryos have a microtubular cage surrounding the nuclei, where microtubules originated from the MTOC (arrow) span the granules (detail). Microtubules were labeled with Clip170- GFP, and granules with Vasa-dsRed. D) Cytoplasmic granules colocalize with microtubular fibers projecting from the nuclear cage (detail). Tubulin expression is also found within the granule (arrowhead) of 11 hpf embryos. Nucleus is depicted by n. E) A close-up view of a germ cell of a 11 hpf embryos reveals flow of cytoplasmic granules not associated with microtubules (arrowhead) into the protrusion (asterisk) while others remain stable in colocalization with microtubules projected from the MTOC (arrow). EXPRESSION / LABELING:
|
|
Granules structure is affected after microtubule disruption. A) Confocal section of germ cells in control embryos exposed to DMSO for 6 hours. Germ cell granules show normal perinuclear localization and distribution into small structures (arrow). B) Confocal section of germ cells in embryos exposed to 1 μg/ml of nocodazole for 6 hours. Nocodazole treated cells exhibit a conglomeration of Vasa positive granules forming a large granule (arrow). C) Embryos exposed to same conditions mentioned above. Vasa-dsRed labels granules, LaminB2-GFP labels nuclear envelop. The experiment shows how after laminB2 network disassembles, individual granules fuse forming bigger structures (asterisk). Cell outline is depicted with white dashed lines. All experiments were done in 11 hpf embryos. |
|
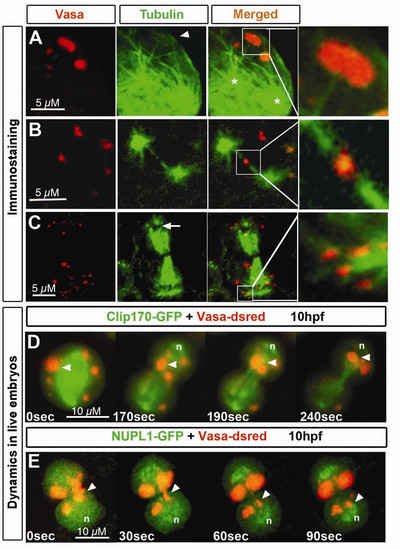
Microtubules localization relative to germ cell granules during different stages of germ cell mitosis. Immunostaining of germ cells undergoing cell division. Granules are labeled with anti-Vasa antibody and microtubules with anti-α-Tubulin antibody. A) During beginning of mitosis, α-Tubulin- expressing granules (arrowhead) appear connected to microtubular fibers (detail) projecting from what appear as centrosomes (asterisks). B) At a later stage of mitosis, when the two presumed centrosomes are located at the poles of the cell, projecting spindle microtubules reach at Vasa and α-Tubulin expressing granules (detail). C) When mitosis is close to termination, granules are enriched around the area where the nucleus reassembles (arrows). Specific colocalization of granules with astral microtubules is shown in detail. D) Germ cell granules (arrowhead) move towards the forming nucleus (n) through a path of spindle microtubules. E) Granules move to the area where the nucleus forms. Nuclear envelope reassembly was visualized by NUPL1. Elongated granules (arrowhead) move from the cytokinetic region to the site of the forming nucleus (n) and re-adopt a spherical shape upon arrival All experiments were performed on 10 hpf embryos. EXPRESSION / LABELING:
|
|
Dynein localize to germ cell granules and is involved in granule fragmentation. A) DynL2-GFP is localized to nucleus, cytoplasm and to germ cell granules. Granule DynL2-GFP localization patterns are diverse, ranging from ring-like structures around large granules (left panel, arrowhead, nucleus is depicted by n), dots within the granules (arrowhead, middle panel) or accumulations can also be observed on the periphery of such structures (arrowhead, right panel). Interphase cells where analyzed in 6 to 10 hpf embryos. Note that Vasa-dsRed and DynL2-GFP colocalization preferentially occurs in the largest granule of the cell. B) Germ cells of 9 hpf embryos expressing DynL2-GFP were analyzed in vivo by epifluorescence microscopy. DynL2-GFP is localized to a large germ cell granule where two centers of high concentration are observed preceding the division of the granule (asterisks in B,C). C) Merge of B together with Vasa-dsRed to visualize the granules. D) Immunostaining of control germ cells of 24 hpf embryos showing the normal number (shown graphically in F) and size (shown graphically in G) of Vasa expressing granules n = 33 cells and 191 granules (G). Dynein inhibition in germ cells of 24 hpf embryos by p50 overexpression reduces the number of granules per germ cell while the size of germ cell granules increases. n = 116 cells and 154 granules (E,G). TTtest for F: p < 1E-8. TTest for G: p < 1E-14. EXPRESSION / LABELING:
|
|
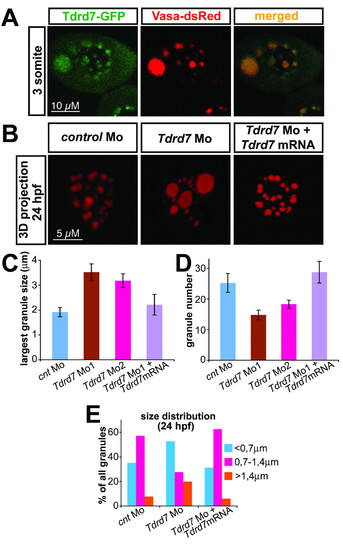
Tdrd7 function is required for proper germ cell granule architecture. A) Tdrd7-GFP fusion protein colocalizes with the germ cell granule marker Vasa-dsRed. A 3-somite stage embryo corresponds to 11 hpf embryo. B) Tdrd7 function is required the formation of uniform normal sized granules. A 3D projection of granules labeled with Vasa antibody in 24 hours old control cells (left panel), in Tdrd7 depleted cells (middle) and in cells from embryos co-injected with the antisense oligonucleotide directed against Tdrd7 and an RNA encoding Tdrd7 that is resistant for the inhibition by the antisense oligonucleotide (right). C) Size of the largest granule in 24 hpf PGC. N = 18 cells for control, 16 for morpholino 1 injected embryos (T-test compared with the control, p < 10-3), 19 for morpholino 2 and 16 cells in which the phenotype was reversed by co-injection of the morpholino resistant Tdrd7 RNA. D) Number of granules in 24 hpf PGC. N = 13 cells for control, 19 cells for morpholino 1 (T-test compared with the control, p < 10-2), 26 cells for morpholino 2 and 13 cells in which the phenotype was reversed by co-injection of the morpholino resistant Tdrd7 RNA., TTest p < 0,003). E) Granule-size distribution is increased in cells knocked down for Tdrd7 as the proportion of the very small and very large granules is increased. N = 223 granules for control, 150 granules for morpholino 1 and 132 granules in which the phenotype was reversed by co-injection of the morpholino resistant Tdrd7 RNA. |
|
granulito morpholino inhibits translation of granulito-yfp mRNA. vasa-dsRed and granulito-yfp mRNAs were coinjected with control morpholino (left panel) or granulito morpholino (right panel). While in the control both Vasa-dsRed and Granulito-YFP are visible, only Vasa-dsRed can be detected in granulito morphants. This demonstrates that the translation of granulito mRNA is efficiently and specifically blocked by the morpholino. |
|
Inhibition of cytokinesis does not interfere with perinuclear localization of granules following nuclear division. Epifluorescence pictures of germ cells labeled with Farnesylated-GFP, H1M-GFP for visualizing plasma membrane, and Vasa-dsRed for germ cell granules. A) Cells expressing RhoAN19 do not undergo cytokinesis and polynucleated cells are observed. Although no cytokinetic ring is formed, granules reach the nuclear envelope (n) and readopt perinuclear localization (arrowhead) after division of the nucleaus. B) Interphase cells show normal perinuclear distribution of germ cell granules in both nuclei. |
|
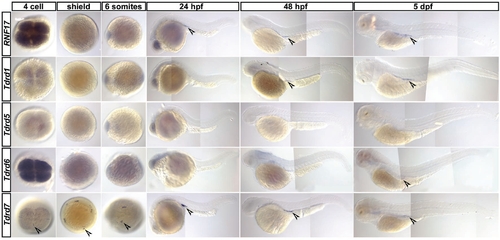
Expression pattern of Tudor domain containing proteins in zebrafish embryos and larvas. In situ hybridization for zebrafish homologs of previously described tudor domain containing genes RNF17, Tdrd1, Tdrd5, Tdrd6 and Tdrd7 at different stages starting from 4cell stage until 5 dpf. For RNF17 germ cell specific expression was observed starting from 24 hpf. For Tdrd1 germ cell specific expression was observed starting from 48 hpf. For Tdrd5 no expression could be observed in the analyzed stages. For Tdrd6 faint staining at the region where germ cells reside is visible at 3 dpf and 5 dpf. Tdrd7 expression is observed specifically at the cleavage furrow of the 4cell stage where the germ plasm is localized and continues to be expressed specifically in the germ cells throughout the analyzed stages. |
|
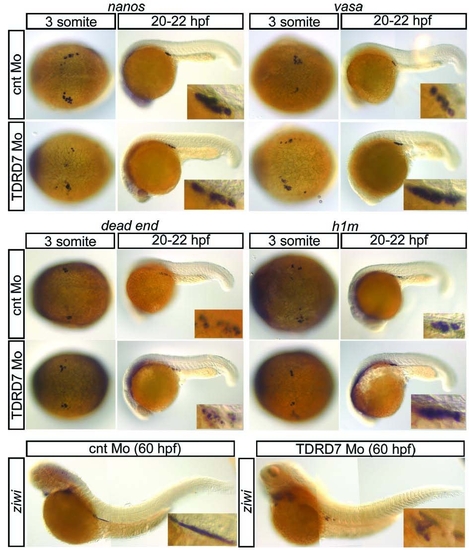
In situ hybridization of PGC markers in Tdrd7 morphants. The early PGC markers nanos, vasa, dead end and h1m are normally expressed in PGCs of Tdrd7 morphants as judged by the comparison to control embryos. ziwi, a marker for PGC differentiation after the migratory stages starts its expression normally in Tdrd7 morphant germ cells. EXPRESSION / LABELING:
|
|
Verification of the Tdrd7 knock down phenotype. A) 3D projection of granules labeled with Granulito-EYFP in 24 hours old control cells (left panel), in Tdrd7 depleted cells (right panel) verifies that Tdrd7 function is indeed required for the formation of uniform normal sized granules. B) Granulito and Vasa colocalize as well in the Tdrd7 morphant situation with each other. C) Coexpression of the GFP fusion to dynein light chain2 and Vasa DsRed in Tdrd7 morphant PGCs shows a comparable dynamic localization of Dynein in germ cell granules as it is known for wildtype PGCs. D) Using Clip170-GFP as a marker for microtubular networks shows normal network for interphase as well as mitotic PGCs. |

Unillustrated author statements PHENOTYPE:
|