- Title
-
Embryonic requirements for ErbB signaling in neural crest development and adult pigment pattern formation
- Authors
- Budi, E.H., Patterson, L.B., and Parichy, D.M.
- Source
- Full text @ Development
|
Defective adult pigment pattern but normal embryonic/early larval pigment pattern of picasso mutants. (A) Wild-type adult pigment pattern of picasso heterozygote. (B) Defective pigment pattern of picasso homozygote. (C,D) Pigment patterns of wild-type and mutant siblings were indistinguishable at 5 dpf. (E-H) Repeated images of wild-type (picasso/+) larvae revealed normal development of initially dispersed metamorphic melanophores that organized into stripes (arrow, E), as well as metamorphic melanophores that developed already at sites of stripe formation. (I-L) picasso mutant larvae develop very few metamorphic melanophores (arrow, L), and instead many embryonic/early larval melanophores (arrowhead, K) persisted into the adult. (E,I) 17 dpf. (F,J) 23 dpf. (G,K) 31 dpf. (H,L) 40 dpf. |
|
Regulative posterior adult stripe formation in the picasso mutant. Adult pigment pattern development of wild-type (A-D) and picasso mutant (E-H) larvae. (A-D) In wild type, most embryonic/early larval melanophores remained in a dorsal position, though a few were incorporated into the adult stripe posteriorly (arrowheads in C,D). (E-H) In picasso mutants, more embryonic/early larval melanophores (e.g. arrowheads in G) were incorporated into an incomplete stripe. Additional metamorphic melanophores differentiated (e.g. arrows in G) where persisting embryonic/early larval melanophores contributed to the adult stripe. (A,E) 14 dpf. (B,F) 16 dpf. (C,G) 20 dpf. (D,H) 24 dpf. PHENOTYPE:
|
|
ErbB gene expression in metamorphosing larvae. (A,B) erbb3 loci were expressed in glia (∼18 dpf). Arrows, glial cells surrounding midbody lateral line. (A) erbb3b. (B) erbb3a. (C) RT-PCR revealed metamorphic melanophore expression of erbb3b and erbb2, but not erbb3a or egfr. All four ErbB genes were expressed in adult fin. mel, melanophore; dct, dopachrome tautomerase (encoding a melanin synthesis enzyme expressed by melanophores and their precursors). dct is more strongly expressed in melanoblasts (present in fin) compared with melanophores (mel). Scale bar: in A, 0.5 mm for A,B. EXPRESSION / LABELING:
|
|
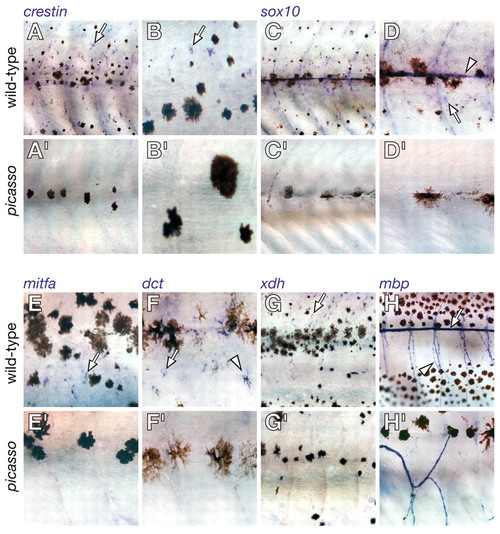
Metamorphic deficiencies for early and late markers of neural crest-derived lineages in picasso mutant larvae. Shown are corresponding regions of the mid-trunk for wild type (above) and picasso mutants during mid-metamorphosis (∼18 dpf). Individual melanophores are more spread in picasso mutants, typical of reduced melanophore densities (Parichy and Turner, 2003b; Parichy et al., 2003). (A,A′) crestin marks neural crest-derived cells in embryos (Luo et al., 2001) and identifies dispersed cells in the hypodermis of metamorphosing larvae (arrow). crestin+ cells were dramatically fewer in picasso mutants. (B,B′) Higher magnification image of crestin+ cells in wild type, and their absence in picasso. (C,C′) sox10 marks non-ectomesenchymal neural crest-derived cells in embryos, including pigment cell and glial precursors (Dutton et al., 2001; Gilmour et al., 2002), and identifies comparable populations in metamorphosing larvae (Parichy et al., 2003). sox10+ cells were fewer in picasso compared with wild type. (D,D′) Higher magnification showing sox10+ cells along the lateral line (arrowhead) and in the hypodermis (arrow) of wild type, but not picasso. (E,E′) mitfa marks melanophore and xanthophore precursors in embryos and is essential for melanoblast specification (Lister et al., 1999; Parichy et al., 2000b). mitfa+ cells (arrow) were numerous in wild type but not in picasso. (F,F′) dct identifies melanophore precursors (arrow) and melanophores (arrowhead) (Kelsh et al., 2000); dct+ cells were reduced or absent in picasso. (G,G′) Xanthine dehydrogenase (xdh) encodes an enzyme in the pteridine synthesis pathway of xanthophores (Parichy et al., 2000b). xdh+ cells (arrow) were numerous in wild type but transiently fewer in picasso. (H,H′) Myelin basic protein (mbp) marks mature glia in the peripheral nervous system (Brosamle and Halpern, 2002; Lyons et al., 2005) and mbp+ cells line the midbody lateral line (arrow) as well as ascending and descending nerve fibers (arrowhead). Although mbp+ cells were present in picasso, they were fewer in number and their associated nerves were misrouted. EXPRESSION / LABELING:
PHENOTYPE:
|
|
Autonomous and non-autonomous roles for erbb3b in pigment pattern metamorphosis. (A) Wild-type → picasso chimeras frequently developed wild-type melanophores in stripes at high density anteriorly (arrows, left) but at lower density in the mid-trunk (small arrows, right; 75% of chimeras developed donor melanophores; chimeras with donor cells and total reared: n=24, 64, respectively). A wild-type midbody lateral line is misrouted as well. (B) Melanophores at high density anteriorly that are either donor-derived (EGFP+) or host-derived (EGFP-). (C) Melanophores in the mid-trunk are more spread, which is typical at low density. In reciprocal picasso → wild-type chimeras, we did not observe donor metamorphic melanophores (n=7, 50). (D) Wild-type → nacre chimeras developed patches of donor-derived metamorphic melanophores that populated stripes (arrow) and scales (84% of chimeras developed metamorphic melanophores; n=75, 155). Persisting embryonic/early larval melanophores (arrowheads) are identifiable by location, large size and browner color (Quigley et al., 2004). (E) picasso → nacre chimeras developed melanophores (arrowheads), but did not develop metamorphic melanophores [79% of chimeras developed embryonic/early larval melanophores or fin melanophores (not shown); n=58, 195]. Donor cells in all chimera combinations contributed at similar frequencies to other derivatives, including muscle, epidermis, eye and neurons of the lateral line. Scale bars: in A, 500 μm; in B, 200 μm for B,C; in D, 1 mm for D,E. |
|
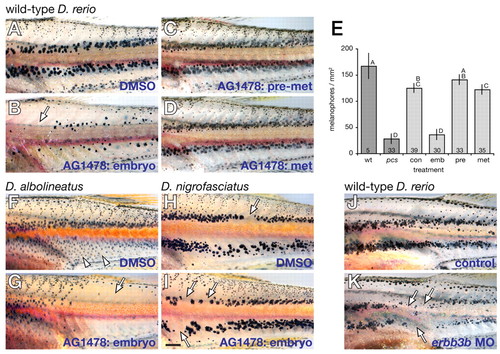
Embryonic requirement for ErbB signaling in pigment pattern metamorphosis. (A) Control wild-type zebrafish treated from embryonic through juvenile stages with DMSO alone. (B) Wild-type treated with AG1478 during embryonic stages (70% epiboly - 4 dpf) showed adult pigment patterns resembling severely affected picasso mutants (arrow). (C,D) Individuals treated with AG1478 during the pre-metamorphic, early larval period (5-14 dpf, C) or throughout metamorphosis (15-28 dpf, D) exhibited pigment patterns indistinguishable from controls. (E) Metamorphic melanophore densities in the mid-trunk (mean±95% confidence intervals) showing similarities between wild-type (untreated), control (con, DMSO-treated) and fish treated with AG1478 during pre-metamorphosis (pre) and metamorphosis (met), as well as similar defects between picasso mutants (pcs) and fish treated with AG1478 as embryos (emb). Letters above bars indicate means that are not significantly different (P>0.05). Numbers within bars are samples sizes. (F,G) D. albolineatus normally develop a more uniform melanophore pattern of metamorphic melanophores (arrowheads in F) compared with D. rerio, and exhibited a severe melanophore deficiency when embryos were treated with AG1478 (G). (H,I) In D. nigrofasciatus, adult stripes largely comprise persisting embryonic/early larval melanophores with occasional gaps (arrow in H), and embryonic treatment with AG1478 had relatively subtle effects (arrows in I). (J,K) Embryonic morpholino knockdown of erbb3b resulted in adult melanophore deficiencies (K) compared with controls (J), providing independent evidence for an early erbb3b-dependence of adult pigment pattern formation. Scale bar: in I, 0.5 mm for A-D,F-I,J,K. PHENOTYPE:
|
|
Treatment of embryos with ErbB inhibitor PD158780 results in adult pigment pattern defect similar to erbb3b null alleles. (A) Treated with DMSO alone. (B) Treated with PD158780 for 2 dpf. Arrow, region deficient for metamorphic melanophores. |
|
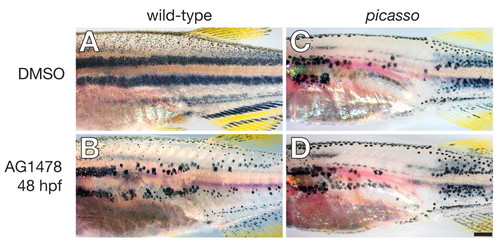
ErbB inhibitor treatment for 48 hpf does not enhance the picasso null phenotype. (A) Wild-type control treated with DMSO. (B) Wild-type treated with AG1478 exhibits a severe pigment pattern defect. (C) picasso mutant control treated with DMSO. (D) picasso mutant treated with AG1478 has a pigment pattern defect indistinguishable from that of the control. Scale bar: in D, 0.5 mm for A-D. PHENOTYPE:
|
|
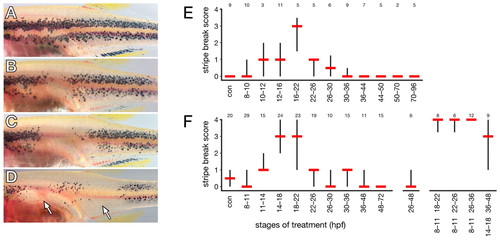
kit mutant reveals critical period for ErbB activity during neural crest migration. Embryos were reared to juvenile stages after treatment with AG1478 as embryos. (A) Normal kit mutant pigment pattern after AG1478 treatment between 26 and 30 hpf (stripe break score=0; for details see Materials and methods). kit mutant adults exhibit about half as many stripe melanophores as wild-type fish and completely lack melanophores over the dorsum and on scales (Johnson et al., 1995). (B,C) Treatment with AG1478 between 14 and 18 hpf results in pattern defects that are moderate (B, stripe break score=3) to severe (C, stripe break score=4). (D) Distinct melanophore-free patches (arrows) in kit mutant treated between 8 and 11 hpf then again between 26 and 30 hpf. (E,F) Quantitation of stripe break defects in kit mutants treated with AG1478 in two separate experiments. Shown are median stripe break scores (red bars) and interquartile ranges (50% of scores, black vertical bars). Numbers above each treatment stage are sample sizes of adult fish analyzed. con, DMSO-treated controls. (E) Initial experiment reveals peak sensitivity between 16 and 22 hpf, with lesser defects extending between 8 and 36 hpf (test of differences in median locations across all treatments, Wilcoxon test χ2 approximation=38.6, d.f.=11, P<0.0001). (F) Second experiment confirms peak sensitivity between ∼14 and 22 hpf (χ2=115.6, d.f.=14, P<0.0001). More severe defects are generated with repeated treatments (right). PHENOTYPE:
|
|
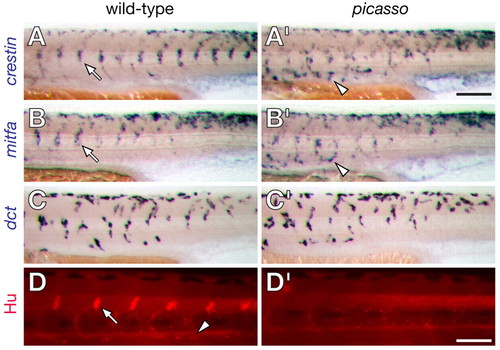
picasso mutant embryos have defects in neural crest morphogenesis. (A,A′) crestin+ cells form segmentally arranged clusters prior to ganglion formation in wild-type embryos (e.g. arrow in A) but appear to migrate past their normal target sites in picasso mutant embryos (e.g. arrowhead in A′). (B,B′) mitfa+ cells in wild-type exhibit some segmental patterning and a defect in picasso mutants similar to that of crestin+ cells. (C,C′) dct+ melanoblast distributions do not differ consistently between wild-type and picasso mutant embryos. (D,D′) Anti-Hu immunoreactivity shows dorsal root ganglia (e.g. arrow in D) and sympathetic ganglia (e.g. arrowhead in D) in wild-type larvae but their absence in picasso mutants. Scale bar: in A′, 800 μm for A-C′; in D′, 600 μm for D-D′. |
|
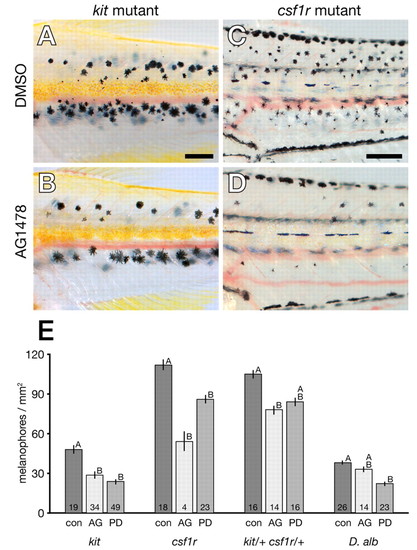
Cryptic ErbB requirements during metamorphosis revealed by sensitized genetic backgrounds. (A) kit mutants (reared with DMSO) develop fewer metamorphic melanophores than wild type. (B) kit mutants reared during metamorphosis with AG1478 have an additional metamorphic melanophore deficiency. (C) csf1r mutants have similar numbers of melanophores to wild type through the middle metamorphic stage shown (Parichy et al., 2000b). (D) csfr1r mutants reared in AG1478 during metamorphosis exhibit a sharp reduction in melanophore numbers, as well as increased larval mortality (not shown). (E) Melanophore densities (±1 s.e.) are moderately or significantly reduced in sensitized backgrounds when treated with AG1478 (AG) or PD158780 (PD) compared with DMSO-only controls (con). Shared letters above bars indicate treatments that are not significantly different from one another (P>0.05) within each genetic background. Treatment sample sizes are shown at the base of each bar. Scale bar: in A, 300 μm for A,B; in C, 300 μm for C,D. PHENOTYPE:
|