- Title
-
Identification of a novel retinoid by small molecule screening with zebrafish embryos
- Authors
- Sachidanandan, C., Yeh, J.R., Peterson, Q.P., and Peterson, R.T.
- Source
- Full text @ PLoS One
|
DTAB causes anterior-posterior axis defects. PHENOTYPE:
|
|
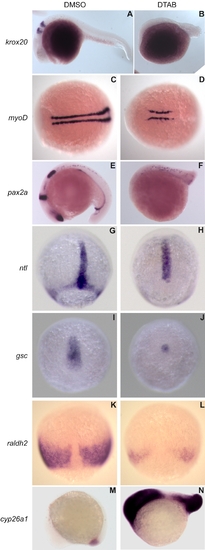
DTAB affects the expression of early developmental markers. EXPRESSION / LABELING:
PHENOTYPE:
|
|
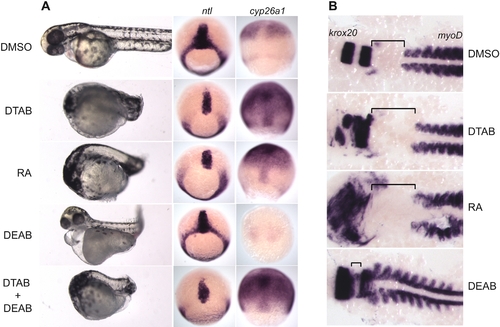
DTAB mimics effects of RA on early development. EXPRESSION / LABELING:
PHENOTYPE:
|