- Title
-
Rasl11b knock down in zebrafish suppresses one-eyed-pinhead mutant phenotype
- Authors
- Pézeron, G., Lambert, G., Dickmeis, T., Strähle, U., Rosa, F.M., and Mourrain, P.
- Source
- Full text @ PLoS One
|
Rasl11b, an atypical cytoplasmic Ras small GTPase, is strongly conserved in Vertebrates. (A) Zebrafish rasl11b encodes a Ras-related small GTPase of 244 amino acids (accession number DQ983377) containing the 5 highly conserved domains (G1–G5, overlined in red) responsible for the guanine nucleotide-dependent molecular switches. Rasl11b has no obvious orthologues in Drosophila melanogaster or Caenorhabditis elegans, but is highly conserved among vertebrates. Note that, in contrast to most of the Ras small GTPases, Rasl11b lacks a COOH-terminal CAAX motif and any known recognition signal for C-terminal lipidation found in Ras proteins such as farnesylation or palmitoylation allowing membrane anchorage. The amino acid positions mutated to create the activated forms Rasl11bS42V and Rasl11bQ82L are indicated with stars. (B) Phylogenic analysis of zebrafish small GTPase proteins. The degree of relatedness is indicated by the length of the vertical lines. Numbers indicate bootstrap support for nodes. Red box: Rasl11b, Rasl11a, Rasl12 and Rerg constitute an uncharacterized branch of Ras proteins devoiish small GTPase proteins. The degree of relatedness is indicated by the length of the vertical lines. Numbers indicate bootstrap support for nodes. d of lipid modification |
|
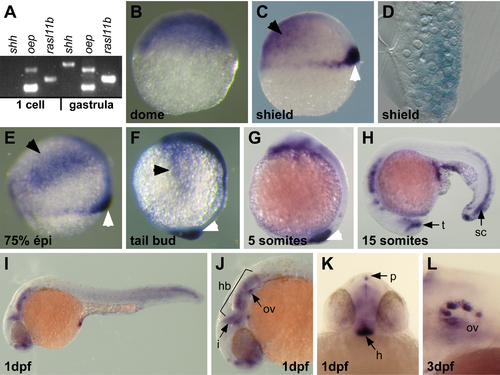
rasl11b expression pattern during zebrafish embryogenesis.(A) RT-PCR analysis showing that rasl11b has a maternal and a zygotic component. RNA extractions have been done before (1 cell stage) and after (gastrula) the midblastula transition, the time of activation of the zygotic transcription. The maternally and zygotically expressed oep gene and the strictly zygotic sonic hedgehog (shh) gene have been used as controls. (B) During the cleavage period (dome stage), rasl11b is ubiquitously expressed. (C) At the onset of gastrulation (shield stage), rasl11b is still detected at the animal pole (Black arrowhead) but is also expressed in a dorso-ventral gradient at the dorsal margin (white arrowhead). This marginal expression overlaps with the mesendodermal territory in zebrafish embryos. (D) Sagittal section. rasl11b transcript accumulates in both hypoblastic and epiblastic dorsal blastomeres. (E, F) rasl11b expression is maintained at the margin throughout gastrulation (white arrowheads). Gastrulae also expressed n. rasl11b transcript accumulates in both hypoblastic and epiblastic dorsal blastomeres. (E, F) rasl11b exprerasl11b mRNA in ectodermal precursors located at the lateral borders of the blastoderm (black arrowheads). (G–K) During somitogenesis and organogenesis, rasl11b is expressed in the tail tip and the spinal cord (sc), and in several head structures such as the hindbrain (hb), the isthmic organizer (i), the otic vesicle (ov), the pineal gland (p), the ventral hypothalamus (h) and the posterior boundary of the telencephalon (t). (J) lateral close up and (K) frontal v EXPRESSION / LABELING:
|
|
Opposite effects of Nodal and Oep on rasl11b expression. (A) Scheme of the Nodal cascade. The Oep coreceptor is necessary for the binding of the Nodal ligand Cyc and Sqt to the TGFβ receptors (containing the type I receptor Taram-A, the zebrafish Alk4 orthologue) that in turn phosphorylate Smad2. Smad2-P is translocated to the nucleus and triggers the transcription of a first set of genes encoding the transcription factors, Mixer, Gata5 and Mezzo. This set is required for the expression of the Sox factor Casanova that in turn initiates the transcription of the endodermal markers sox17 and axial/foxa2. (B–M, Dorsal view of gastrulae save C, I, sagittal sections and G, lateral view) (B, C) In wild-type (WT) embryos rasl11b is expressed at the animal pole (arrows) and at the dorsal margin (black arrowheads). (D) This marginal expression is lost in MZoep mutants (star), devoid of maternal and zygotic oep, consequently devoid of Nodal signaling, and so, unable to form most of the mesendoderm. (E, F) rasl11b dorso-pression is lost in MZoep mutants (star), devoid of maternal and zygotic oep, consequently devoid of Nodal marginal expression is normal in the cyclops Nodal mutant and largely reduced in the MZsquint Nodal mutant. (G) Activation of the Nodal signal by injection of a constitutively activated form of the Nodal type I receptor Taram-A (tar*) leads to a duplication of the rasl11b marginal expression domain (likely by inducing a second organizer, white arrowheads) (H, I) In Zoep mutants, devoid of zygotic oep, this mesendodermal expression domain is extended (white arrowheads). (J–M) A large series of embryos expressing different levels of Nodal signal was generated by injecting between blastula cells increasing doses of the recombinant Lefty protein, a Nodal pathway extracellular inhibitor. Here, only four representative doses are displayed. A progressive decrease of rasl11b marginal expressi |
|
rasl11b knock down induces a specific curly down phenotype in zebrafish. (A) Scheme of the rasl11b transcript. The Morpholino targeted sequences are represented by thin red lines. The red boxes correspond to the G1 to G5 domains described in Fig. 1. (B–D) Lateral view of 1 dpf embryos. Both rasl11b MO-ATG and rasl11b MO-GT induce a curly tail down phenotype when injected in WT. This phenotype can be rescued by the coinjection of rasl11b mRNA resistant to MO knock down, rasl11bMOr (D). (E) In vitro translational assay showing the efficient knock down effect of the rasl11b MO-ATG on wild-type rasl11b mRNA compared to the MO resistant rasl11bMOr mRNA. (F,G) rasl11b splice-blocking morpholino (rasl11b MO-GT) knocks down endogenous rasl11b mRNA. RT-PCR analysis detected two splice variants that were cloned and sequenced. Sequence comparison revealed that the splice variant (1) resulted from aberrant splicing to an upstream cryptic slice donor site twn endogenous rasl11b mRNA. RT-PCR analysis detected two splice variants that were cloned and sequenced. Sequence comparison revealehat is present in intron 2, r PHENOTYPE:
|
|
Rasl11b inhibits endoderm and PP formation in an Oep deficient background. (A–D) Frontal views of 1 dpf wild-type embryo and Zoep embryos. Depending on the quantity of oep mRNA accumulated during oogenesis, Zoep embryos display different levels of anterior structure development: from absence of lens and forebrain (red class, B), via a cyclopia phenotype (green class, C) to two separate retinae (blue class, D, very rare). In each clutch, these categories can have slightly different proportions depending on the female, but a large majority of Zoep embryos do not even develop one retina. Whatever females (n>10) were used for the rasl11b knock down experiments, a drastic rescue (>70%) of the forebrain formation was always observed, and a large category of embryos with two retinae appeared. (E) Cumulated numbers for each class from 5 independent rasl11b MO-ATG knock down experiments. (F-H) forkhead7 (fkd7) expression pattern in 1 dpf embryos. Gut defects (white arrowheads) are also rescued (black arrowheads) ass from 5 independent rasl11b MO-ATG knock down experiments. (F-H) forkhead7 (fkd7) exprein Zoep embryos injected by rasl11b MO-ATG. (I-L) 1 dpf embryos lateral views. Rasl11b constitutively activated forms (Rasl11b*) prevent head formation and disturb the antero-posterior axis formation in Zoep and oep+/- but not in wild-type embryos (not shown). zrx2 is expressed in the retina, krox20 in rhombomeres 3 and 5, myod is expressed in the somitic mesoderm. (M–V) dorsal view of late gastrulae. rasl11b knock down rescues expression of the prechordal plate marker goosecoid (gsc) and the endodermal marker sox17 in Zoep where EXPRESSION / LABELING:
PHENOTYPE:
|
|
rasl11b interacts specifically with oep but does not affect the Nodal/Smad2 transduction pathway. (A) The MZsqt, MZoep, Zoep, bon and cas mutants have a clear reduction of endodermal cells and so were used to quantify the putative impact of rasl11b knock down at different steps/levels of the nodal pathway. The Zoep mutant was the only one rescued by the rasl11b MO-ATG injection. This rescue was abolished by co-injection of rasl11b MO-resistant mRNA (rasl11bMOr). Error bars indicate standard deviation. (B, C) It is impossible to generate clutches of 100% Zoep embryos, and because one embryo cannot give enough material for both immunoblot and genotyping experiments, 100% Zoep-like mutant clutches were produced by injecting clutches of 100% MZoep eggs with low doses of wild-type oep mRNA. Half of them were then co-injected with rasl11b MO-ATG. Each batch was split in two, one used fant clutches were produced by injecting clutches of 100% MZoep eggs with low doses of wild-type oep mRNA. Half ofor phosphorylated Smad2 (Smad2-P) level analysis, the second for endodermal cell number count (assayed by sox17 in situ hybridization). Proteins were detected by western blotting using a Smad2-P antibody. Note that increasing doses of wild-type oep mRNA were correlated with an increase of Smad2-P and sox17 endodermal cell number, whereas co-injection with rasl11b MO-ATG increased endodermal cell number without generating more Smad2-P (Error bars indicate standard PHENOTYPE:
|