- Title
-
The coxsackie and adenovirus receptor (CAR) is required for renal epithelial differentiation within the zebrafish pronephros
- Authors
- Raschperger, E., Neve, E.P., Wernerson, A., Hultenby, K., Pettersson, R.F., and Majumdar, A.
- Source
- Full text @ Dev. Biol.
|
Expression pattern of CAR mRNA in the zebrafish pronephros. (A and B) CAR mRNA expression was detected in early development of the glomerular precursors (arrowhead), as seen by in situ hybridization in 24 hpf embryos. The inset shows the glomerular precursor expression from the dorsal view. (C and D) At 48 hpf, CAR mRNA was detected in the pronephric glomerulus (arrowhead) and gut (arrow). Embryos are visualized with the anterior to the left and dorsal to the top. |
|
CAR protein expression in zebrafish pronephros. Immunohistochemical co-stainings of CAR with the tight junctional protein ZO-1 were performed on transversal sections of the pronephros from a 96 hpf zebrafish larvae. (A) CAR and ZO-1 were detected in parietal epithelium (pe) of the Bowman's capsule that surrounds the pronephric glomerulus (pg) and in the gut (g). Podocyte and endothelial expression of ZO-1 was also detected in the pronephric glomerulus. (B) In the pronephric tubules (pt), CAR and ZO-1 co-localize to epithelial cell–cell contacts close to the lumen (asterisks in panels B and C), and this apical localization was verified by co-staining of CAR and the basolateral marker Na+/K+-ATPase alpha1 subunit (C). The notochord (n) is indicated in panel A for orientation and DAPI was used to visualize the nuclei (blue). Scale bar, 50 μm. EXPRESSION / LABELING:
|
|
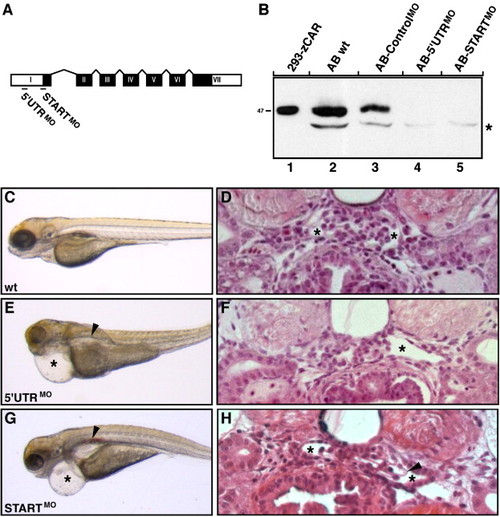
CAR morphants display phenotypes associated with kidney failure. (A) Two antisense oligonucleotide morpholinos (MO), one complementary to the 5′ UTR region (5′ UTRMO) and the other to the region harboring the ATG site (STARTMO), were designed to bind to CAR mRNA and block translation. The seven exons of the CAR gene, encoding the CAR protein, are shown in black boxes with roman letters. The 52UTR and 32UTR regions are marked with white boxes. Exons and introns are not drawn to scale. (B) The morpholino knockdown efficiency was verified by immunoblot analysis on total protein lysates isolated from five individual zebrafish embryos at 48 hpf. Lysates were separated under reducing conditions and CAR was detected using the anti-CAR SIV antibodies. The CAR protein decreased to undetectable levels in both CAR morphants (lanes 4 and 5) as compared to wild type (wt) embryos (lane 2) and controlMO morphants (lane 3). Exogenously expressed zebrafish CAR (zCAR) was used as control for the molecular size (lane 1). The position of the molecular weight marker (kDa) is indicated to the left and asterisk indicates non-specific staining. (C, E and G) Wild type (wt) larvae and CAR morphants were visualized by regular light microscopy at 96 hpf. While wild type larvae developed normally (C), injection of both CAR specific morpholinos results in morphants with cardiac edema (asterisks) and renal cysts (arrowheads) (E and G). (D, F and H) Transversal sections through the pronephric glomeruli of wild type larvae and CAR morphants at 96 hpf. Both CAR morphants (F and H) display enlarged Bowman′s space (asterisks) when compared to wild type larvae (D). In addition, a less densely organized glomerular tuft is seen in the STARTMO morphant (H, arrowhead). No gross histological differences in surrounding tissues could be observed between wild type larvae and CAR morphants (D, F and H). Data shown are a representative larvae/morphant for the indicated time point. |
|
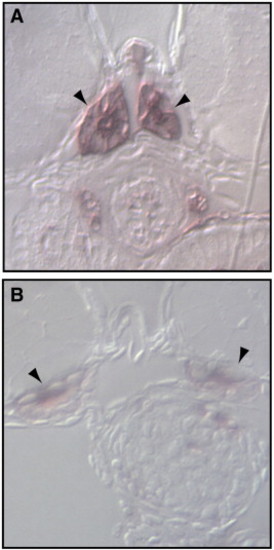
Ultrastructural analysis of CAR morphant pronephros reveals fused podocyte foot processes and abnormalities of the epithelium. Comparison of the ultrastructural morphology of control and CAR morphants at 96 hpf. (A and B) While the pronephric glomerulus of the control morphants (A) has well shaped and evenly spaced podocyte foot processes that are connected with slit diaphragms (arrowheads), the CAR morphants display a reduced number of slit diaphragms, as seen as fused podocyte foot processes (asterisks, B). The epithelial microvilli of the pronephric tubules are markedly reduced or missing in the CAR morphants (D and F), when compared to control morphants (C and E). In addition, CAR morphants display an increased distance between the neighboring plasma membranes (arrowheads) in pronephric tubular (H) and gut (J) epithelium, when compared to control morphants (G and I, respectively). Scale bar, 250 nm, except in panel F, 25 μm. PHENOTYPE:
|
|
CAR morphants display loss of endogenous alkaline phosphatase activity in pronephric tubules. Transversal sections through pronephric tubules (arrowheads) of control and CAR morphants at 96 hpf were incubated in NBT/BCIP. Reduction of endogenous alkaline phosphatase activity in apical duct cells was observed in CAR morphants (B) as compared to controls (A). PHENOTYPE:
|
|
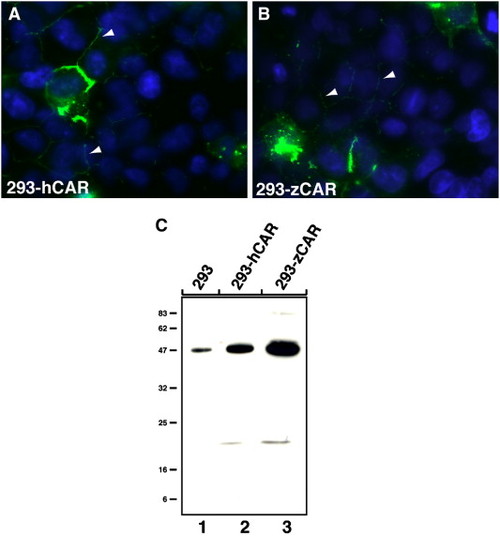
Characterization of the anti-CAR SIV antibodies against human and zebrafish CAR. Immunofluorescent and immunoblot analysis of human (hCAR) and zebrafish (zCAR) CAR transfected QBI-293A cells (293). The anti-CAR SIV antibodies detected exogenously expressed hCAR and zebrafish zCAR at cell–cell contacts as well as at the cell-surface of transfected 293 cells (A and B, respectively). Immunoreactivity of the antibody against endogenously expressed hCAR in 293 cells is indicated with arrowheads (A and B). (C) The anti-CAR SIV antibodies react with an approximately 47 kDa protein band in cell lysates from exogenously expressed hCAR and zCAR in transfected 293 cells (lanes 2 and 3, respectively). Endogenously expressed hCAR was used as control for the molecular size (lane 1). The position of the molecular weight marker (kDa) is indicated to the left. |
|
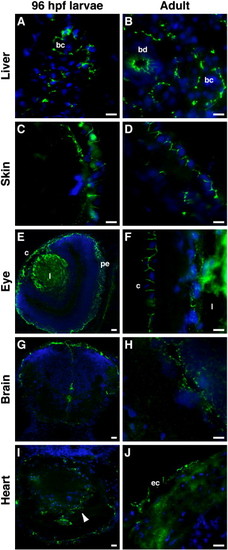
CAR is mainly found at epithelial cell–cell contacts in larvae and adult zebrafish. Immunohistochemical staining was performed, using the anti-CAR SIV antibodies, on cryo-sections from 96 hpf larvae and adult zebrafish. In the liver, CAR expression was found at cell–cell junctions between hepatocytes, forming the bile canaliculi (bc), in both larvae (A) and adult (B) zebrafish. CAR was also detected in adult bile ducts (bd; B). In the skin, CAR localized to epithelial cell–cell contacts (C and D). In the eye, CAR expression was found in the cornea (c) in both the larvae (E) and adult (F) zebrafish. CAR was also detected in the pigmented epithelium (pe) of the larvae (E). Immunoreactivity of the anti-CAR SIV antibody was observed in the lens (l; E and F). Both in the larvae (G) and adult (H) zebrafish brain, CAR expression was detected in cells lining the ventricles and the outer surface of the brain. In the larval heart (I, arrowhead), CAR expression was detected in the tissue, while the expression of CAR in the adult heart was found at cell–cell contacts in the epicardium (ec in J). DAPI was used to visualize the nuclei (blue). Scale bar, 50 μm. |
|
CAR localizes to epithelial tight junctions in the zebrafish larvae. Sub-cellular localization of CAR and the tight junctional proteins ZO-1 and cingulin was performed on 96 hpf zebrafish larvae. The stainings were analyzed using high resolution confocal microscopy. CAR was found to co-localize with ZO-1 (A) and cingulin (B) at epithelial tight junctions of the skin. The nuclei are visualized with DAPI (blue). The images were analyzed in the x–y (upper panels) and x–z (lower panels) planes. Optical magnification, x 63. EXPRESSION / LABELING:
|

Unillustrated author statements |
Reprinted from Developmental Biology, 313(1), Raschperger, E., Neve, E.P., Wernerson, A., Hultenby, K., Pettersson, R.F., and Majumdar, A., The coxsackie and adenovirus receptor (CAR) is required for renal epithelial differentiation within the zebrafish pronephros, 455-464, Copyright (2008) with permission from Elsevier. Full text @ Dev. Biol.