- Title
-
The role of survivin in angiogenesis during zebrafish embryonic development
- Authors
- Ma, A.C., Lin, R., Chan, P.K., Leung, J.C., Chan, L.Y., Meng, A., Verfaillie, C.M., Liang, R., and Leung, A.Y.
- Source
- Full text @ BMC Dev. Biol.
|
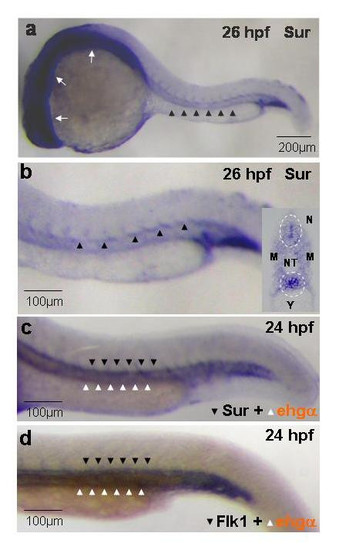
Whole-mount in-situ hybridization showing the expression of survivin in zebrafish embryos. (a, b): Survivin is expressed diffusely in the developing central nervous system (white arrows) and the axial vasculature (arrowheads) at 26 hpf. Similar expression patterns were seen at 56 hpf (not shown). (b, insert): Coronal section of stained embryos at 26 hpf showing preferential expression of survivin at the vicinity of the dorsal aorta and the developing neural tube (circled). (c, d): Double in-situ hybridization showing remarkably similar expression pattern of survivin (c) and flk1 (d) (blue, dark arrowheads) in relation to that of embryonic hemoglobin-α (brown, white arrowheads). Pictures are representative of at least three separate experiments. NT: Neural Tube; M: Myotome; N: Notochord; Y: Yolk sac extension. EXPRESSION / LABELING:
|
|
Effects of survivin knock-down on embryonic development. (a, b): Wild-type (WT) embryos injected with random sequence morpholino at 22 hpf (a) and 48 hpf (b). (c, d): Embryos injected with a combination of SurATG (3 ng) and SurUTR morpholinos (3 ng) (SurATG+UTR) at 1–4 cell stage. Noted that while there was no significant morphological changes at 22 hpf, most of the embryos injected with SurATG+UTR morpholinos at 48 hpf showed a "characteristic phenotype" with reduced head and eye size (circled) and a mildly curved tail (arrowheads). Similar phenotypes were also seen in embryos injected with either SurATG or SurUTR morpholinos at various doses but not in WT embryos injected with random sequence. Insert (d) showed a severe phenotype at 48 hpf characterized by severely deformed embryos which did not survive beyond 48 hpf. These embryos were not included in the analysis. Each picture is representative of at least three experiments. (e): The dose-dependence of either SurATG, SurUTR or SurATG+UTR morpholinos. Optimal response was observed when embryos were co-injected with 3 ng of each MO (SurUTR+ATG). Results were expressed as mean ± S.E.M. In each experiment, MOs at different doses were injected into the same batch of embryos and were scored at the same time. More than 40 embryos have been injected at each dosage. PHENOTYPE:
|
|
Effects of survivin knock-down on angiogenesis and circulation. (a, b): Confocal microscopy of Tg(fli1:EGFP)y1 embryos either uninjected (a) or injected with SurUTR+ATG morpholinos (b). Noted the aberrant sprouting of the inter-segmental vessels (ISV) (arrowheads), the absence of vertebral arteries (arrows) and the failure to form the dorsal anastomotic vessels (DLAV) in the SurUTR+ATGMO embryos. AC: Axial circulation. Noted that the dorsal aorta and posterior cardinal vein in the axial circulation could not be distinguished based on the resolution provided. (c, d): Fluorescent images in Tg(fli1:EGFP)y1 embryos showing failure to develop the sub-intestinal vessels (SIV) in SurUTR+ATGMO embryos. (e-h): Microangiographic pictures in uninjected (e, g) and SurUTR+ATGMO embryos (f, h) showing defective vasculatures in ISV, DLAV, optical veins (OV) and inner optic circle (IOC). N: Notochord; AC: Axial circulation. (i, j): Whole-mount TUNEL assay in embryos injected with random sequence MO (i) and SurUTR+ATG-MO (j) showing positive staining in the area of developing neural tube and brain (white arrows) as well as at the vicinity of the axial circulation (white arrowheads) in the SurUTR+ATGMO embryos. N: Notochord; Y: Yolk sac extension. Embryos were examined at 48 hpf except (c) & (d) which were examined at 96 hpf. More than 20 embryos have been examined in each experiment. |
|
Effect of survivin knock-down was gene-specific. (a-d): Injection of 5'UTR-survivin:GFP plasmids gave rise to green fluorescence in a mosaic pattern in 79.7 ± 9.4% (a, c) which was totally abolished by co-injection with SurUTR+ATG-MO (b, d). (e): Uninjected Tg(fli1:EGFP)y1 embryos at 48 hpf. (f): Defective sprouting of inter-segmental vessels, similar to those seen in SurUTR+ATGMO embryos, could be recapitulated by injecting embryos with survivin morpholino targeting the splice-site junction (g). (h): Molecular targeting was confirmed using RT-PCR showing survivin gene in injected embryos contained a larger transcript compared with uninjected ones. (i-k): Defective angiogenesis in SurUTR+ATGMO embryos could be rescued by co-injecting with survivin mRNA. (i): Uninjected embryos. (j): SurUTR+ATGMO embryos. (k): SurUTR+ATGMO embryos co-injected with survivin mRNA. (i): Histogram showing average number of embryos with normal inter-segmental vessels (ISV) in three separate experiments. All embryos were oriented anterior (left) to posterior (right). PHENOTYPE:
|
|
Regulation of survivin expression by vascular endothelial growth factor (VEGF) at 96 hpf. (a): Sub-intestinal vessels in uninjected Tg(fli1:EGFP)y1 embryos. (b): Injection of human VEGF (2 ng) gave rise to ectopic angiogenesis (arrows). There was no observable ectopic angiogenesis in the ISV (c): Axial and inter-segmental vessels in untreated Tg(fli1:EGFP)y1 embryos. (d): Tg(fli1:EGFP)y1 embryos treated with VEGF tyrosine kinase receptor inhibitor (VEGFTKRI, 25 μmol/L) showing defective sprouting of inter-segmental vessels. (e): Injection of survivin mRNA did not reverse the defects seen in VEGFTKRI treated embryos. (f): Histogram showing the average survivin mRNA expression (expressed in fold-change) in untreated and VEGFTKRI treated embryos as well as in embryos injected with human VEGF. Results expressed in mean ± S.E.M. (n = 3 experiments using at least 20 embryos per experiments). When the three groups of data were compared using Kruskal-Wallis Test, p-value = 0.016. When the data of uninjected vs VEGFTKRI treated embryos were compared using Mann-Whitney U Test, p-value = 0.037. |

Unillustrated author statements |