- Title
-
WNT8 and BMP2B co-regulate non-axial mesoderm patterning during zebrafish gastrulation
- Authors
- Ramel, M.C., Buckles, G.R., Baker, K.D., and Lekven, A.C.
- Source
- Full text @ Dev. Biol.
|
Wnt8 and BMP2b are required to maintain axial/non-axial polarity. Morphology of bud stage embryos. Genotypes are indicated above each column. A–D: dorsal views. F–I: lateral views, dorsal right. Arrows in panels A–C indicate the width of the notochord when visible. Double arrows in panels F–I indicate the length of the A/P axis, asterisks indicate rostral edge of anterior axial mesoderm. Note the shortened axis of wnt8 MO embryos (C, H) and the posterior displacement of the axis in wnt8 MO; swr/+ embryos (D, I). wnt8 MO; swr/swr embryos display a large mass of cells at the animal pole (arrows in E and J) and do not have a recognizable D/V polarity. PHENOTYPE:
|
|
Wnt8 and BMP2b are necessary to repress anterior and posterior axial mesoderm and to maintain non-axial mesoderm. In situ hybridization for gsc (anterior axial mesoderm, A–E), myoD (adaxial mesoderm, F–J), and flh (posterior axial mesoderm, K–O) at bud stage. Genotypes are indicated above each column. Main panels A–E: lateral views, dorsal right. Insets: dorsal view, anterior up. F–O: dorsal views, anterior up. Insets: posterior view, dorsal up. Note that the slightly widened adaxial domain in swr mutants (G) and the progressive widening of the axial domain as wnt8 MO embryos have a reduced dosage of bmp2b (C–E, H–J, M–O). EXPRESSION / LABELING:
|
|
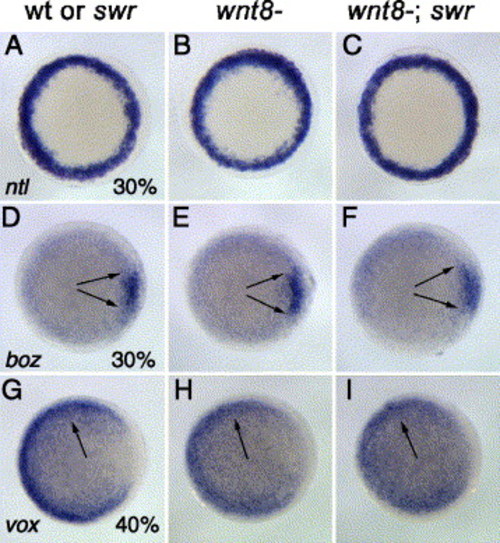
Wnt8 and BMP2b are required to maintain non-axial identity after 40% epiboly. In situ hybridizations for ntl (A–C), boz (D–E), and vox (G–I). Genotypes and stages are indicated. The pan-mesoderm marker ntl is induced normally in wnt8; swr double mutants (C). The dorsal marker boz is expressed in a wild-type pattern in wnt8- (E) and wnt8-; swr embryos (F) at 30% epiboly. vox is expressed at similar levels in wnt8 single mutants and wnt8; swr double mutants at 40% epiboly (H, I). All panels: animal views, dorsal right. EXPRESSION / LABELING:
|
|
Both wnt8; swr and vent; vox double mutants display expansion of axial mesoderm, but only wnt8-; swr embryos show loss of non-axial mesoderm. In situ hybridizations for chd (A–F), flh (G–L), eve1 (M–R), cdx4 (S–W), tbx6 X–C′), tbx24 (D′-I′), and ved (J2-O′). Genotypes are indicated above each column. All embryos are at shield stages except panels X–C′ (60% epiboly) and D′–I′ (70% epiboly). Both wnt8; swr (D, J) and vent; vox (F, L) double mutants show a loss of axial mesoderm repression, although flh expansion is less severe in vent; vox mutants than in wnt8; swr mutants (arrow in panel L, compare to panel J). All non-axial–mesoderm markers (eve1, cdx4, tbx6, tbx24, and ved) are strongly reduced or absent in wnt8; swr double mutants (P, V, A′, G′, M′) but are still expressed in vent; vox mutants (R, W, C′, I′, O′). Asterisks in panels D′-I′ represent the dorsal limit of tbx24 staining. (A–W) Animal views, dorsal right. (X–O′) Lateral views, dorsal right, anterior up. EXPRESSION / LABELING:
|
|
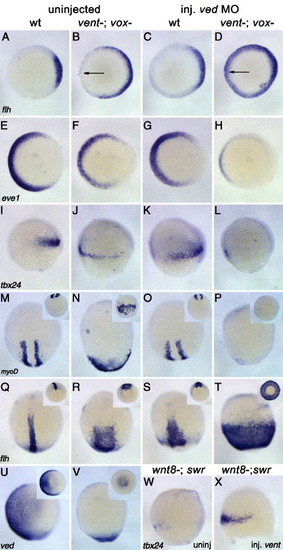
Knockdown of Ved in vent; vox mutants replicates the phenotype of wnt8; swr mutants. In situ hybridization for flh (A–D, Q–T), eve1 (E–H), tbx24 (I–L, W–X), myoD (M–P), and ved (U–V). Genotypes are indicated. A–H: shield stage, animal views, dorsal right. I–L, W–X: 70% epiboly, lateral views, dorsal right. M–V: bud stage, dorsal view (M–T) or lateral views, dorsal right (U–V). Insets M–T: vegetal views, dorsal up. Insets U–V: vegetal view, dorsal right. flh expression in vent; vox mutants is expanded ventrally but not in a robust fashion (arrow in panel B, inset in panel R). Injection of ved MO in vent; vox mutants results in a stronger expansion of flh (arrow in panel D, inset in panel T). The expression of the non-axial mesoderm markers eve1, tbx24, and myoD is strongly reduced in the vent/vox/ved triple knockdown (H, L, P). At bud stage, vent; vox mutants still display ved expression at the margin but not in the future epidermis (U, V). Injection of vent RNA in wnt8; swr double mutants does restore some tbx24 expression but does not rescue the phenotype to wild-type (W, X, compare to I). EXPRESSION / LABELING:
|
|
Vent and Vox function redundantly to repress myoD expression. A–H: In situ hybridization for myoD at bud stage. Dorsal views, anterior is up. Insets show dorso-vegetal views, dorsal up. Injected morpholinos are indicated above each panel, all morpholinos were injected at 5 ng/nL each. The percentage of treated embryos displaying the phenotype shown in each panel is indicated. Horizontal bars indicate width of adaxial myoD+ domain. Vent and Vox double knockdown recapitulates the Dfst7 phenotype (B), while knockdown of Vent or Vox individually results in a mild phenotype (C, D). ved-MO-injected embryos display a wild-type myoD pattern (E). Double morphant combinations (B, F, G, H) show that the loss of Vox + Vent or Vox + Ved gives rise to a ventrally expanded myoD (arrow in insets). EXPRESSION / LABELING:
|
|
Characterization of wnt8 splice blocking MOs. (A) Structure of the wnt8 locus. Boxes denote exons, black shading represents non-coding regions. wnt8 orf1 and orf2 are the two wnt8 coding regions. Black lines above exons indicate positions of splice blocking MO sequences. Arrows indicate primers used for RT-PCR characterization. (B) RT-PCR analysis of wnt8 splice blocking efficiency at shield stage. Lanes 1–4: PCR using primers 1 and 2. Lanes 5–8: PCR using primers 3 and 4. Lanes 9–12: PCR using primers 1 and 5. Lane 1, 5, 9: RNA was prepared from ∼ 80 embryos injected with orf1 splice blocking MOs. Lanes 2, 6, 10: RNA was prepared from ~ 80 embryos injected with orf2 splice blocking MOs. Lanes 3, 7, 11: RNA was prepared from ∼ 80 embryos injected with all MOs. Lanes 4, 8, 12: RNA was prepared from wild type embryos. Molecular weight standard sizes are indicated (kb). Arrows indicate the size of the expected wild-type products. Note that the orf1 splice blocking MOs prevent complete splicing of the orf1 transcripts (lane 1) but not orf2 transcripts (lane 2) and orf2 splice blocking MOs do not block orf1 splicing (lane 5) but efficiently block orf2 splicing (lane 6). Note the almost complete absence of wild-type full-length transcripts when four splice blocking MOs are injected (lane 11). (C) Rescue of the splice-blocker-induced phenotype by wild-type RNA. Since the splice blocking MOs bind to unspliced exon/intron junctions, wild-type RNA is not affected by the MOs. Left: examples of phenotypes observed in wild-type embryos injected with wnt8 MOs or in embryos injected with both wnt8 MOs and wild-type wnt8 orf1 RNA. C1–C5 are dorsalization classes described by Mullins et al. (1996) where C5 is the strongest dorsalized phenotype and C1 is characterized by the loss of the ventral tail fin. Right: graph of rescue experiment results. In this experiment, wnt8 RNA (concentration = 10 ng/μl) was injected into embryos previously injected with MOs. RT-PCR Methods: approximately 80 embryos from each treatment were homogenized in Trizol (Invitrogen) and processed according to the manufacturer′s instructions. 0.5–1.0 μg RNA was used for first strand cDNA synthesis with Thermoscript primed with an oligo-dT primer (Invitrogen). Forty PCR cycles were performed for the results shown in this figure. EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 287(2), Ramel, M.C., Buckles, G.R., Baker, K.D., and Lekven, A.C., WNT8 and BMP2B co-regulate non-axial mesoderm patterning during zebrafish gastrulation, 237-248, Copyright (2005) with permission from Elsevier. Full text @ Dev. Biol.