- Title
-
A unique role for 6-O sulfation modification in zebrafish vascular development
- Authors
- Chen, E., Stringer, S.E., Rusch, M.A., Selleck, S.B., and Ekker, S.C.
- Source
- Full text @ Dev. Biol.
|
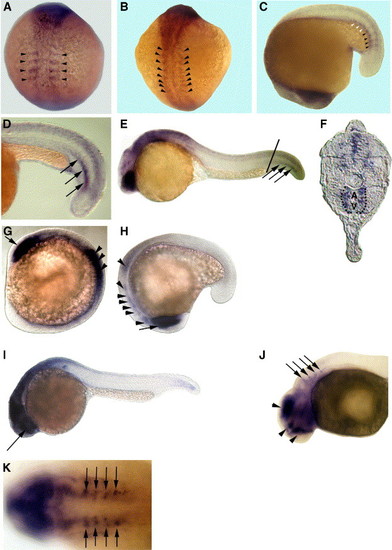
Embryonic expression patterns of zebrafish HS6ST-1 and HS6ST-2 mRNA. (A) 4-somite stage. (B) 10-somite stage. (C) 16-somite stage. Zebrafish HS6ST-2 is expressed in the forming somites (arrowheads in panels A–C). HS6ST-2 expression is down-regulated in more differentiated somites (white arrowheads in C). (D) At 22-somite stage (20 hpf), zebrafish HS6ST-2 is expressed in a stripe of cells in somitic mesoderm (arrows). (E) At 24 hpf, expression of zebrafish HS6ST-2 is detected in the eyes, the brain and ventral medial somites in the tail. (F) A transverse section of the embryo in (E). Zebrafish HS6ST-2 expression is detected in the ventral medial somites and cells surrounding the dorsal aorta (A) and posterior cardinal vein (V) in the tail (as outlined by the dashlines). The level of the section is indicated by the line in (E). (G) At 10-somite stage, zebrafish HS6ST-1 is expressed in the optic vesicles (arrow) and the neural tube (arrowheads). (H) At 16-somite stage, zebrafish HS6ST-1 is expressed in the developing eyes (arrow), the tegmentum, the forming rhombomeres and the neural tube (arrowheads). (I) At 24 hpf, expression of zebrafish HS6ST-1 is detected in the eyes (arrow), the brain and the neural tube. (J) At 33 hpf, expression of zebrafish HS6ST-1 is detected in the diencephalon, the telencephalon, the tegmentum (arrowheads) and in four bilateral patches of cells at the level of the dorsal rhombomeres (arrows). (K) Dorsal view of the embryo in (J), showing the four bilateral stripes of cells in the rhombomeres (arrows). (A–B) Dorsal view, anterior to the top. (C, H) Lateral view, anterior to the bottom. (D–E, I–J) Lateral view, anterior to the left. (G) Lateral view, anterior to the top left. (K) Anterior to the left. EXPRESSION / LABELING:
|
|
Zebrafish HS6ST-2 is essential for branching morphogenesis of the caudal vein in zebrafish embryos. (A) Tg(fli-1:EGFP) embryo at 48 hpf under FITC illumination with inset at the lower left corner showing a region of the venous plexus. (B) Angiogram of the Tg(fli-1:EGFP) embryo in (A) under TRITC illumination. (C–D) Tg(fli-1:EGFP) embryo injected with 5 ng of HS6ST-2 MO2 (C) and the angiogram of the same embryo (D) showing reduced branching as indicated by large loops (arrowheads) in the caudal vein plexus. (E–F) In the more severe case, the venous plexus in a Tg(fli-1:EGFP) embryo fails to achieve the complexity as observed in Tg(fli-1:EGFP) embryos. Instead, the formation of a cavernous vessel (arrowheads) is observed. (G) A summary graph showing results of injections using two non-overlapping morpholinos against the zebrafish HS6ST-2 gene. Both oligos generate similar cardinal vein defects including formation of large loops and cavernous vessels with high penetrance. In contrast, HS6ST-1 MO (column 3) does not generate a significant percentage of embryos with CV defects. CV, cardinal vein. N denotes the number of embryos analyzed. ±SEM. The lines in panels A, C and E indicate the segments of venous plexus displayed in insets. |
|
Co-injection of zebrafish HS6ST-2 expression construct alleviates the caudal vein defects in HS6ST-2 morphant embryos. (A) Uninjected Tg(fli-1:EGFP) embryo at 33 hpf under FITC illumination. (B) HS6ST-2 MO2-injected embryo with defective caudal vein branching as indicated by the formation of a cavernous vessel (arrows). (C) Embryo co-injected with HS6ST-2 MO2 and a mixed solution of HS6ST-2 DNA and DsRed DNA, showing the restoration of the venous plexus (arrows) under FITC illumination. DsRed is introduced as a tracer to facilitate the identification of DNA-injected embryos. (D) DsRed expression in the same co-injected embryo showing mosaic expression pattern in the tail under TRITC illumination. (E) Overlay of panels C and D, showing DsRed expressing-cells, correlating with areas in the restored venous plexus (arrowheads). (F) Summary of four independent co-injection experiments. The test DNA (HS6ST-2 expression construct) or the control DNA (DsRed expression construct) was injected into embryos separately injected with HS6ST-2 MO2. The introduction of HS6ST-2 protein by the DNA injection alleviates the caudal vein defects in HS6ST-2 MO2-injected embryos (compare column 2 to column 1). Asterisk (*) denotes statistical significance with P < 0.0005. In contrast, a separate injection of control DNA into HS6ST-2 MO2-injected embryos does not affect the frequency of caudal vein defects in HS6ST-2 MO2-injected embryos (compare column 3 to column 1; P > 0.2). N indicates the total number of embryos scored. ±SEM. |
|
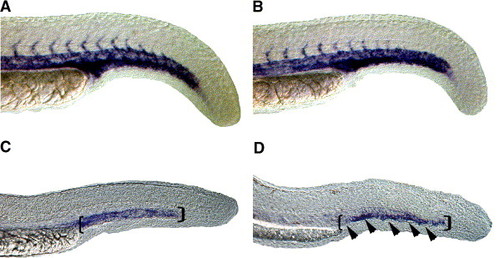
Expression of late endothelial markers in the region of forming venous plexus is reduced in HS6ST-2 morphants. Expression of flk-1 as shown by whole-mount in situ hybridization in a wild-type embryo (A) and a HS6ST-2 MO-injected embryo (B) at 24 hpf. Normal expression of flk-1 is observed in HS6ST-2 MO-injected embryos at 24 hpf (n = 19). However, at 30 hpf, the expression domain of the late endothelial marker, tie-1 (D; 81% ± 8%, n = 40, ±SEM) is reduced in the region of caudal vein (area indicated by brackets and arrowheads) in HS6ST-2 MO-injected embryos compared to wild-type embryos (C). |
|
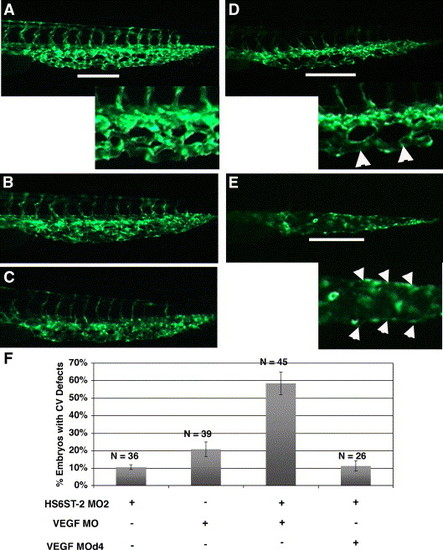
HS6ST-2 and VEGF-A interact during caudal vein plexus formation in vivo. (A) Uninjected Tg(fli-1:EGFP) embryo at 33 hpf under FITC channel, with the inset displaying an enlargement of a segment in the venous plexus. (B) Embryo injected with 0.5 ng of HS6ST-2 MO2 displaying a venous plexus without any obvious defect. (C) Embryo injected with 1 ng of VEGF-A MO, displaying a venous plexus without any obvious defect. (D–E) Embryo co-injected with HS6ST-2 MO2 and VEGF-A MO displaying defective branching morphogenesis of the venous plexus as indicated by the formation of large loops (arrowheads in inset of D) and a single cavernous vessel with no branching (arrowheads in inset of E). The lines in A, D and E indicate regions in the venous plexus that are displayed in the insets. (F) A summary of two independent experiments is shown. Embryos co-injected with HS6ST-2 MO2 (0.5 ng) and VEGF-A MO (1 ng) exhibit a more than additive increase in the frequency (±SEM) of embryos with branching defects in the caudal vein plexus (column 3) compared to embryos injected with HS6ST-2 MO2 or VEGF-A MO only. In contrast, no synergy is observed in the embryos co-injected with HS6ST-2 MO and a four-base mismatch MO (d4, 1 ng) against VEGF-A (column 4). |

Unillustrated author statements |
Reprinted from Developmental Biology, 284(2), Chen, E., Stringer, S.E., Rusch, M.A., Selleck, S.B., and Ekker, S.C., A unique role for 6-O sulfation modification in zebrafish vascular development, 364-376, Copyright (2005) with permission from Elsevier. Full text @ Dev. Biol.