- Title
-
Zebrafish acvr2a and acvr2b exhibit distinct roles in craniofacial development
- Authors
- Albertson, R.C., Payne-Ferreira, T.L., Postlethwait, J., and Yelick, P.C.
- Source
- Full text @ Dev. Dyn.
|
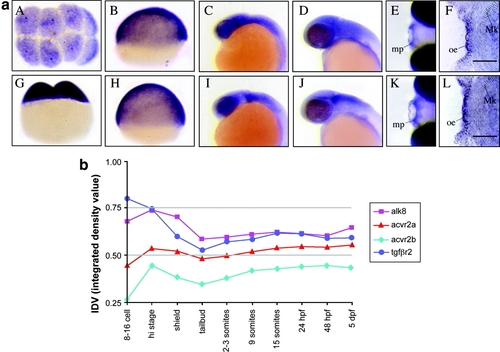
Developmental expression of transforming growth factor-beta (TGFβ) family member receptors. a: Whole-mount in situ hybridization (WISH) analysis of acvr2a (A-F) and acvr2b (G-L). Positive mRNA expression is indicated by purple color. A, E, and K are dorsal views; F and L are flat-mounted ventral views; the remaining panels are lateral views, with anterior to the left and dorsal to the top. Both acvr2a and acvr2b are expressed in early cleavage and epiboly stage embryos (A, B, and G, H, respectively). At 24 and 48 hours postfertilization (hpf), acvr2a expression is restricted to cranial neural tissues (C and D, respectively). At these stages, acvr2b exhibits a more discrete expression pattern, restricted to the midbrain-hindbrain boundary (mhb) and hindbrain (I and J). By 96 hpf, both acvr2 genes exhibit discrete expression in the maxillary processes (mp, E and K) and in the oral epithelium (oe, F and L). Mk, is Meckel′s cartilage. b: Densitometric analysis of reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of acvr2a, acvr2b, alk8 and tgfbr2 gene expression. RT-PCR products specific for each receptor mRNA were generated at indicated developmental times, and size fractionated by gel electrophoresis. Integrated density values (IDV), the absolute sum of pixilated area of each RT-PCR product, were generated from digitally imaged ethidium bromide-stained RT-PCR product normalized to actin controls. Scale bar = 50 μm in F, L. |