- Title
-
Otolith matrix proteins OMP-1 and Otolin-1 are necessary for normal otolith growth and their correct anchoring onto the sensory maculae
- Authors
- Murayama, E., Herbomel, P., Kawakami, A., Takeda, H., and Nagasawa H.
- Source
- Full text @ Mech. Dev.
|
Developmental expression of zebrafish omp-1 (zomp-1) and otolin-1 (zotolin-1). zomp-1 in situ hybridization at 14-somite stage (A) and 24 hpf (B). zomp-1 transcripts are detected only in the otic vesicles (arrowheads). (C) Magnification of the otic vesicle at 24 hpf. zomp-1 expression is more intense in the ventro-posterior part (arrowhead). (D) zomp-1 in situ hybridization at 72 hpf. The expression level of zomp-1 is lower at the anterior and posterior maculae (am and pm, outlined by dotted lines) than in the non-sensory parts of the epithelium (arrowheads). A dotted line indicates the outline of the otic vesicle. (E) Otic vesicle of a 26-somite embryo labeled with an anti-OMP-1 antibody. Both otoliths are slightly labeled. (F) At 35 hpf, both otoliths are heavily stained with the anti-OMP-1 antibody. The ventro-lateral epithelium posterior to the anterior macula is also labeled (arrowhead). (G–I) zotolin-1 in situ hybridization at 72 hpf; three increasingly deeper focal planes of the same ear; zotolin-1 transcripts are not detected in the anterior (G) nor in the posterior macula (H), but at the dorsal and ventral sides of the latter (I, arrowheads); am, anterior macula; pm, posterior macula. In panels (E) and (F), two photos at different focal planes are juxtaposed for focusing the two otoliths. All panels show lateral views, anterior to the left, dorsal to the top. Scale bar in (C) indicates 25 μm in panels (C) to (I). EXPRESSION / LABELING:
|
|
Otolith phenotypes observed in live control, zomp-1 and zotolin-1 MO-injected embryos by DIC videomicroscopy. Embryos were injected with 4 ng MO. (A–C) 28-somite stage; otolith seeding occurs normally in control MO (A), zomp-1 MO (B) and zotolin-1 MO (C) injected embryos. (D–F) Magnification of the anterior otolith and two tethering kinocilia shown in (A–C). (G–K) At 35 hpf, the otoliths of zomp-1 MO (I) and zotolin-1 MO (J) injected embryos are slightly smaller, and closer to each other, respectively, than those of control MO-injected embryos (H); the anterior otolith of zotolin-1 MO-injected embryos does not stay in contact with the anterior macula (arrowhead in J); (G, K) Anterior macula, otolith and tethering kinocilia of the zomp-1 MO ear shown in (I) and zotolin-1 MO ear shown in (J). (L–N) At 55 hpf, zomp-1 MO and zotolin-1 MO injection cause a ‘small otoliths’ phenotype (M) and a ‘fused otoliths’ phenotype (N), respectively. (O) Magnification of the cilia of the anterior macula shown in (N). At 72 hpf (P–S), the otoliths of control MO (P) and zotolin-1 MO (R) injected embryos keep growing, especially the posterior otolith, while both otoliths of zomp-1 MO-injected embryos stay small (Q). (S) Magnification of the cilia of the anterior macula shown in (R). In panels (A–C), (H), (I), (L), (M), (P) and (Q), the pictures of posterior otoliths are inlayed, from a deeper focal plane, onto the main pictures, which are focused on the anterior otoliths. Through the panels, anterior is to the left, dorsal to the top. Scale bar in (A) indicates 25 μm in (A–C), in (D) indicates 10 μm in (D–F), in (H) indicates 25 μm in (H–J, L–N, P–R), in (G) indicates 10 μm in (G), (K), (O) and (S). |
|
Whole-mount immunostaining of the otoliths of wild type and MO-injected embryos. (A, D, G, J) Live embryos; all other panels show fixed, immunostained embryos. (A–C) Wild-type embryos at 27 hpf; (B) zOMP-1 is detected in both otoliths and in the ventro-lateral epithelium posterior to the anterior macula (arrowheads), but zOtolin-1 is not observed yet (C). (D–O) Embryos at 72 hpf. In control MO-injected embryos, anti-OMP-1 (E) and anti-Otolin-1 (F) antibodies recognize both otoliths. (G–I) zomp-1 MO-injected embryos; zOMP-1 is effectively knocked down (H); the anti-Otolin-1 antibody fails to detect zOtolin-1 in the small otoliths (I). (J–L) zotolin-1 MO-injected embryos; zOtolin-1 is not detected (K) while the anti-OMP-1 antibody reacts strongly with the fused otoliths (L). (M–O) Pre-immune serum shows non-specific staining (arrowheads) of the sensory hair cells of the anterior macula (M), posterior macula (N) and anterior and lateral cristae (O). In panels (A–G), the posterior otoliths were inlayed from a deeper focal plane onto the main pictures, which are focused on the anterior otoliths. Dotted lines outline otoliths in panels (H), (I) and (K) and the posterior macula in (N). Through the panels, anterior is to the left, dorsal to the top. Scale bar in (A) and (O) indicates 25 μm in (A–N) and (O), respectively. |
|
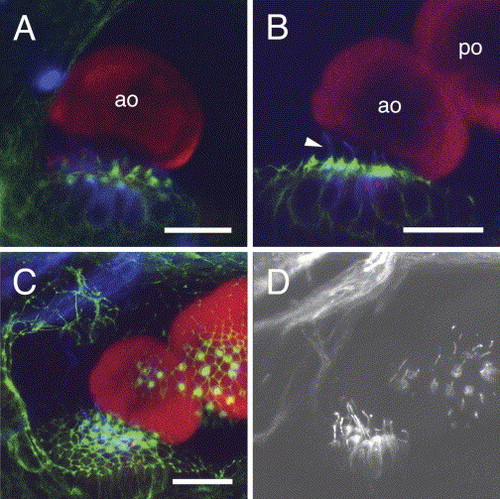
Relationship of the fused otoliths in zotolin-1 MO-injected embryos to the corresponding sensory maculae. Triple-labeling with anti-OMP-1 (red), anti-acetylated tubulin (blue) antibodies, and phalloidin (green). Confocal fluorescence images at 72 hpf. Phalloidin (green) reveals the hair (stereocilia) bundles of the sensory cells and the contours of cells through their actin cortex. Acetylated tubulin (blue, false color) is found in the kinocilia and in the apical cytoplasm of the sensory hair cells (Riley et al., 1997). (A) Anterior macula and otolith of a control MO-injected embryo, lateral view. (B–D) zotolin-1 MO-injected embryo, with fused otoliths. (B) and (C) are the same z-stacks (3D reconstruction available upon request). (B) Anterior macula and anterior otolith with the fused posterior otolith; note the long kinocilia (arrowhead). (C) Projection of the confocal series along the z-axis (22 images, taken every 2 μm). Both maculae are visible through their hair bundles, in relation to both otoliths. (D) Far-red (blue-coded in A–C) fluorescence channel extracted from the image shown in (C), to show the acetylated tubulin immunostaining of kinocilia at both maculae. ao, anterior otolith; po, posterior otolith. Dorso-lateral view, anterior to the left, dorsal to the top. Scale bars, 20 μm. |
|
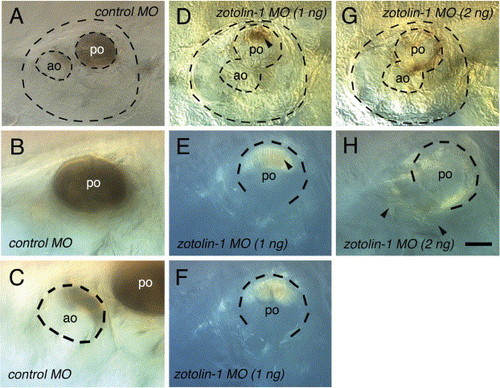
Spontaneous decalcification of the otoliths in fixed control and zotolin-1 MO-injected embryos. Embryos (four for each condition) were fixed at 7 dpf, kept in PBT at 4 °C, and observed 7 days later. All four control embryos still showed a complete posterior otolith and a portion of anterior otolith in both ears, as shown in (A–C). In contrast, 5/8 ears of the four embryos injected with 1 ng zotolin-1 MO had no visible mineralized otolith material left, and 3/8 had only a portion of posterior otolith, as shown in (D–F, arrowhead in D, E). Finally, 8/8 ears of embryos injected with 2 ng zotolin-1 MO had no mineralized otolith material left at all (G, H). Instead, sparse fiber-like material was spread through the otic cavity (arrowheads in H). Scale bar in (H) indicates 25 μm in (B, C, E, F, H). |

Unillustrated author statements EXPRESSION / LABELING:
|
Reprinted from Mechanisms of Development, 122(6), Murayama, E., Herbomel, P., Kawakami, A., Takeda, H., and Nagasawa H., Otolith matrix proteins OMP-1 and Otolin-1 are necessary for normal otolith growth and their correct anchoring onto the sensory maculae, 791-803, Copyright (2005) with permission from Elsevier. Full text @ Mech. Dev.