- Title
-
Gli function is essential for motor neuron induction in zebrafish
- Authors
- Vanderlaan, G., Tyurina, O.V., Karlstrom, R.O., and Chandrasekhar, A.
- Source
- Full text @ Dev. Biol.
|
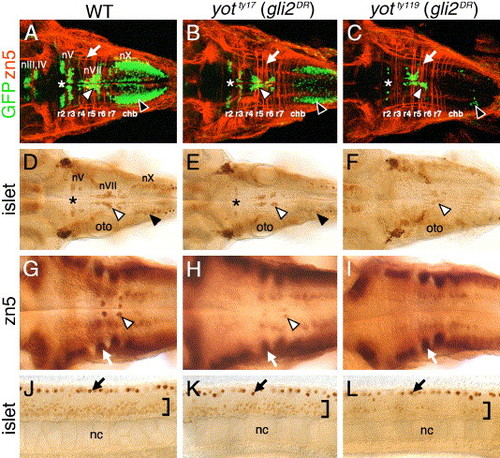
Cranial and spinal motor neuron development is affected in you-too (yot) mutants. Panels A–I show dorsal views of the hindbrain, and panels J–L show lateral views of the trunk, with anterior to the left. Asterisks in A–E indicate the location of nV motor neurons in rhombomere 2. Panels A–C are composite confocal images of embryos and identify GFP-expressing cranial motor neurons in the fluorescein channel, and zn5 antibody-labeled commissural neurons and axons at rhombomere boundaries (arrows) in the rhodamine channel. (A–C) In a 48-h post-fertilization (hpf) wild-type embryo (A), the nIII and nIV somatic motor neurons are located in the midbrain, the trigeminal motor neurons (nV; asterisk) in r2 and r3, the facial motor neurons (nVII; white arrowhead) in r5, r6, and r7, and the vagal motor neurons (nX; black arrowhead) in the caudal hindbrain (chb). The nV neurons are reduced in number in yotty17 mutants (B), and almost absent in yotty119 mutants (C), and, similarly, the nX neurons (black arrowheads) exhibit progressively severe losses in yotty17 and yotty119 mutants. While nVII neurons (white arrowheads) are also reduced in number in both mutant alleles, the reduction is less severe than those for nV and nX neurons. (D–F) At 36 hpf, the islet antibody labels hindbrain motor neurons in characteristic locations (arrowheads, asterisk; see panel A for details) in wild-type embryos (D), and these neurons are found in progressively reduced numbers in yotty17 (E) and yotty119 (F) embryos. (G) In a 48-hpf wild-type embryo, the zn5 antibody labels axons and cell bodies of the nVI abducens motor neurons in rhombomeres 5 and 6 (arrowhead), and the commissural neurons at rhombomere boundaries (arrow). (H and I) In both yot mutants, the commissural neurons (arrows) develop normally, while the nVI neurons are reduced in number in yotty17 mutants (H), and absent in yotty119 mutants (I). (J–L) At 36 hpf, the islet antibody labels motor neurons in the ventral spinal cord (bracket) in wild-type embryos (J), and these neurons are found in progressively reduced numbers in yotty17 (K) and yotty119 (L) embryos. The strongly labeled cells in the dorsal spinal cord (arrows) are Rohon-Beard sensory neurons. oto, otocyst; nc, notochord. EXPRESSION / LABELING:
|
|
Hh signaling is significantly normal in rhombomere 4 of yot mutants. Panels A, B, E–H, and M–S show lateral views of hindbrain, with anterior to the left. Panels C and D show dorsal views with anterior to the left. Panels I–L show cross sections of the hindbrain (top is dorsal) at the indicated rhombomere levels. (A–D) In 21 hpf wild-type embryos (A and C), nk2.2 is expressed (arrowhead) in the ventral neural tube at all axial levels. In yotty119 mutants (B and D), nk2.2 expression (arrowhead) is virtually absent at all axial levels, except in rhombomere 4 (r4), delineated by the expression of krox20 in r3 and r5. Within r4 (D), the nk2.2 expression domain is slightly reduced compared to wild-type. (E–H) In 21 hpf wild-type embryos, ptc1 (E) and net1a (G) are expressed (arrowheads) at all axial levels in the ventral neural tube. In yotty119 mutants, ptc1 expression (F) is greatly reduced, with significant expression retained in r4 (arrowhead). Similarly, while net1a expression is substantially reduced in the yotty119 mutant hindbrain (H), it is significantly normal in r4 (arrowhead). (I–L) In rhombomere 4 (r4), ptc1 expression (arrowheads) in the ventral neural tube is comparable between wild-type (I) and yot mutants (J). In contrast, ptc1 expression in r6 (K) (arrowhead) is greatly reduced in yot mutants (L), while its expression in paraxial mesoderm (arrows) appears normal. Asterisk, notochord. (M–O) While nk2.2 is specifically expressed in r4 of mutants (N, arrowhead), nk2.2 expression in all rhombomeres, including r4 (arrowhead), is completely absent in wild-type and mutant embryos treated with cyclopamine (O). (P) In a control 30 hpf wild-type embryo treated with EtOH from 6 hpf, net1a expression is normal, with elevated expression in r4 (arrowhead). (Q) In a wild-type embryo treated with cyclopamine (CyA) from 6 hpf, net1a expression is completely absent at all axial levels. (R) In an embryo treated from 18 hpf, net1a is mostly expressed in r4 (arrowhead). (S) In an embryo treated from 24 hpf, net1a expression is mostly normal, with prominent expression in r4 (arrowhead). Asterisks in P–S mark the anterior end of the notochord in r4. EXPRESSION / LABELING:
|
|
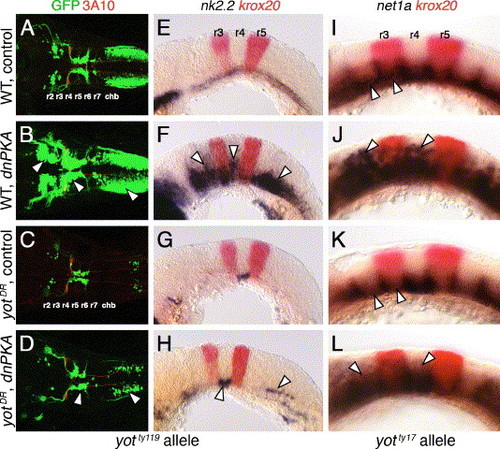
Hh-regulated events in yotty119 mutants are resistant to ectopic Hh pathway activation. Anterior is to left in all panels. Panels A–D show dorsal views, and panels E–L show lateral views. A–D are composite confocal images of embryos, and identify GFP-expressing branchiomotor neurons (green), and 3A10 antibody-labeled Mauthner neurons and axons in rhombomere 4 (red). (A and E) In control wild-type embryos, nk2.2 is expressed ventrally at all axial levels at 21 hpf (E), and branchiomotor neurons are found at their characteristic locations at 36 hpf (A). (B and F) In dnPKA RNA-injected wild-type embryos, nk2.2 is expressed at high levels, and at ectopic locations (arrowheads) in all rhombomeres (F). Branchiomotor neurons are also greatly increased in number (arrowheads), and found at ectopic locations at all axial levels (B). (C and G) In control yotty119 mutants, nk2.2 expression is missing throughout the hindbrain, except within r4 (G), and, concomitantly, there is a severe loss of branchiomotor neurons at all axial levels, except nVII neurons, which originate in r4 (C). (D and H) In dnPKA RNA-injected yotty119 mutants, nk2.2 expression is increased within its normal ventral domain in r4, and slightly within the caudal hindbrain (H, arrowheads). There is a corresponding increase in the number of branchiomotor neurons originating in r4 and the caudal hindbrain (arrowheads), but there is no effect on motor neurons in other rhombomeres (D). (I–L) In a control 21 hpf wild-type embryo (I), net1a is expressed ventrally at all axial levels (arrowheads), with dorsal expansion at rhombomere boundaries. In a dnPKA RNA-injected wild-type embryo (J), net1a expression is expanded dorsally (arrowheads) at all axial levels. In a control yotty17 mutant (K), net1a expression is reduced in all rhombomeres except r4 (arrowheads; compare to I). In a dnPKA RNA-injected yotty17 mutant (L), net1a expression is expanded dorsally in all rhombomeres including r2 and r4 (arrowheads). EXPRESSION / LABELING:
|
|
Hh signaling and Gli1 function are essential for branchiomotor neuron development. Panels A–F and G–O show dorsal and lateral views, respectively, of the hindbrain with anterior to the left. Asterisks in G–O mark the cerebellum. (A) In a 48-hpf wild-type embryo, the nIII, nIV, nV, nVII, and nX motor neurons are found in their characteristic positions. (B–C) In slow muscle-omitted (smu; B) and detour (dtr; C) mutants, GFP-expressing cranial motor neurons are almost completely missing, while the zn5-labeled axons at rhombomere boundaries are unaffected. (D) In a 21-hpf wild-type embryo, nk2.2 is expressed throughout the ventral neural tube at all axial levels. (E–F) In smu (E) and dtr (F) mutants, nk2.2 expression is lost, while krox20 expression in r3 and r5 is unaffected. (G–I) In a 24-hpf wild-type embryo (G), ptc1 is expressed in the ventral hindbrain, while it is expressed diffusely at a reduced level in dtr mutants (I), and not expressed at all in smu mutants (H). (J) In a 30-hpf wild-type embryo, net1a is expressed in the ventral hindbrain and at rhombomere boundaries (arrowhead). (K) In smu mutants, net1a expression is mostly lost, with residual expression in the floor plate (fp). (L) In dtr mutants, net1a expression in the ventral hindbrain is greatly reduced, but expression at rhombomere boundaries (arrowhead) is unaffected. (M–O) In a 21-hpf wild-type embryo (M), gli1 is expressed in the ventral two-thirds of the neural tube, while its expression is greatly reduced in smu mutants (N), and is unaffected in dtr mutants (O). The anterior tip of the notochord (nc) is at the level of r4, and is indicated by arrows. EXPRESSION / LABELING:
|
|
Gli2 contributes to spinal motor neuron induction. Panels A–D show dorsal views, and panels E–L show lateral views of the hindbrain (E–J) and spinal cord (K and L), with anterior to the left. Asterisks in A–D indicate the position of the nIII and nIV motor neurons in the midbrain (A and B), and their absence (C and D). Arrowheads in A–D indicate the nV neurons in r2. (A and E) In control wild-type embryos, nk2.2 expression (E) and organization of cranial motor neurons (A) are identical to those described previously. (B and F) In gli2 MO-injected wild-type embryos, expression of nk2.2 (F) and development of cranial motor neurons (B) are identical to control embryos. (C and G) In control yotty119 mutants, the patterns of loss of nk2.2 expression (G) and cranial motor neurons (C) are similar to those described previously. (D and H) In gli2 MO-injected yotty119 mutants, nk2.2 expression is significantly restored in anterior rhombomeres and the caudal hindbrain (H, arrowheads). Concomitantly, the number of nV neurons (arrowhead) and nX neurons is significantly increased (D), and the pattern looks similar to that in control wild-type embryos. (I and K) In control dtrte370 mutants, net1a is expressed at low levels in the ventral hindbrain, and at higher levels at rhombomere boundaries (I). In the mutant spinal cord (K), islet antibody-labeled motor neurons (arrowhead) are found in similar numbers to wild-type embryos. (J and L) In gli2 MO-injected dtrte370 mutants, net1a expression in the hindbrain (J) shows no significant change (loss). In contrast, the number of motor neurons in the ventral spinal cord (arrowhead) is slightly but reproducibly reduced in MO-injected mutants (L). EXPRESSION / LABELING:
|
|
Gli1 contributes to spinal motor neuron induction. Panels A–D show dorsal views, and panels E–L show lateral views, with anterior to the left. (A, E, and I) In an uninjected 36 hpf wild-type (yot+/+ or +/-) embryo, islet antibody labeling (A) reveals the characteristic organization of the nV neurons in r2 (asterisk), the nVII neurons (white arrowhead), the nX neurons (black arrowhead), and the patterned expression of net1a (E) in the ventral hindbrain and at rhombomere boundaries (arrowheads). Spinal motor neurons (arrowhead, I) are found in characteristic numbers. (B, F, and J) In a yotty119+/- heterozygote injected with gli1 MO, nV and nVII neurons are reduced in number (asterisk, arrowhead in B), nX neurons are absent, and net1a expression is significantly reduced in the hindbrain (arrowheads, F). Spinal motor neurons are slightly reduced in number (arrowhead, J). (C, G, and K) In an uninjected yotty119 mutant, nV (asterisk, C), nX, and spinal motor neurons (arrowhead, K) are greatly reduced in number, while the nVII neurons (arrowhead, C) show a moderate reduction. Net1a expression is significantly reduced in the hindbrain (arrowheads, G). (D, H, and L) In a yotty119 mutant injected with gli1 MO, branchiomotor (D) and spinal motor neurons (L) are completely absent. Net1a expression (arrowheads, H) is also severely reduced in the hindbrain. The strongly labeled cells in the dorsal spinal cord (I–L) are Rohon-Beard sensory neurons, which are unaffected by these treatments. EXPRESSION / LABELING:
|
|
Gli3 plays a role in branchiomotor and spinal motor neuron induction. Panels A–D and I–L show dorsal views of the hindbrain, and panels E–H and M–P show lateral views of the spinal cord, with anterior to the left. (A and E) In an uninjected 36 hpf wild-type (dtr+/+ or +/-) embryo, islet antibody labeling reveals the characteristic organization of the nV neurons in r2 (asterisk), the nVII neurons (white arrowhead), and the nX neurons (black arrowhead) in the hindbrain (A), and the characteristic distribution of motor neurons (arrowhead, E) in the ventral spinal cord. (B and F) In a wild-type sibling injected with gli3 MO, the nV (asterisk), nVII (white arrowhead), and nX (black arrowhead) neurons in the hindbrain (B), and spinal motor neurons (arrowhead, F) are reduced in number. (C, D, G, and H) In an uninjected dtr mutant (C), branchiomotor neurons are essentially absent, and this phenotype is maintained in a gli3 MO-injected dtr mutant (D). In contrast, spinal motor neurons are moderately reduced in number in a gli3 MO-injected dtr mutant (arrowhead, H) compared to an uninjected dtr mutant (arrowhead, G). (I, J, M, and N) In an uninjected wild-type (yot+/+ or +/-) embryo, branchiomotor neurons are found in characteristic numbers (I; see A for details), and their numbers are moderately reduced in a gli3 MO-injected wild-type sibling (J). Similarly, spinal motor neurons are reduced in number in a gli3 MO-injected wild-type embryo (arrowhead, N) compared to an uninjected wild-type sibling (arrowhead, M). (K, L, O, P) In an uninjected yot mutant (K), most branchiomotor neurons, except nVII neurons (arrowhead), are greatly reduced in number or absent, and the number of nVII neurons is slightly decreased in a gli3 MO-injected yot mutant (L). In contrast, spinal motor neurons are greatly reduced in number in a gli3 MO-injected yot mutant (arrowhead, P) compared to an uninjected yot mutant (arrowhead, O). oto, otocyst. |
|
Gli2 expression is affected in the forebrain, but not in the hindbrain, of Hh pathway mutants. All panels show cross sections of the neural tube at the indicated axial levels, with dorsal at the top. Embryos were processed for islet1 (purple); gli2 (red) (A–D, G–J) or nk2.2 (purple); gli2 (red) (E, F) two-color in situs. (A–D) In r4 of a wild-type embryo (A), islet1-expressing motor neurons (arrowhead) are located just ventral to the gli2 expression domain. Similarly, the few islet1-expressing neurons in yot mutants (arrowhead) are located ventral to the gli2 expression domain (B), and gli2 expression does not extend into the ventral-most neural tube in dtr (C) and smu (D) mutants. (E and F) In r5, while nk2.2-expressing cells (arrowhead) are found immediately adjacent to the floor plate and notochord (arrow) in a wild-type embryo (E), nk2.2 expression is absent in yot mutants (F), but the gli2 expression domain does not expand ventrally. (G–J) At the forebrain level, the islet1-expressing epiphysial neurons (arrowhead) are located at the roof of the neural tube. In a 21-hpf wild-type embryo (G), gli2 expression is limited to the dorsal two-thirds of the neural tube (bracket). In contrast, in yotty119 (H), dtrte370 (I), and smub641 (J) mutants, the gli2 expression domain is expanded ventrally to different extents (brackets). EXPRESSION / LABELING:
|
|
Hh signaling is required before 18 hpf for the induction of branchiomotor neurons. Panel A shows a lateral view, and panels F–I show dorsal views of the hindbrain, with anterior to the left. (A) In a 15-hpf wild-type embryo, gli2 is expressed at all axial levels throughout the dorsoventral extent of the hindbrain. The asterisk marks low level of fgf3 expression at the mid-hindbrain boundary, which was used to orient embryos for sectioning (see Materials and methods for details). (B and C) Cross-sections (dorsal is up) showing that gli2 is expressed in the ventral aspects of the neural tube in rhombomeres 5 and 6, but at lower levels than in the dorsal neural tube. nc, notochord. (D and E) Quantification of embryos with normal numbers of GFP-expressing nV motor neurons in r2 (D) and nVII neurons in r4–r7 (E) following cyclopamine (CyA) treatment beginning at the times indicated (hpf). There is no effect on nV neuron number in r2 when CyA treatment is initiated at 12 hpf or later (2 experiments; 20 embryos per experiment). There is no effect on nVII neuron number when CyA treatment is initiated at 18 hpf or later (2 experiments). (F) In a 48-hpf wild-type embryo treated with ethanol (EtOH) from 6 hpf, islet antibody labeling reveals that the number and organization of branchiomotor neurons, including nV (arrow), nVII (white arrowhead) and nX (black arrowhead), are unaffected. (G) In an embryo treated with CyA from 6 hpf, islet-labeled branchiomotor neurons are absent. (H) In an embryo treated with CyA from 12 hpf, nV neurons in r2 are mostly present (arrow; light staining), nVII neurons are greatly reduced in number (arrowhead), and nX neurons are absent. (I) In an embryo treated with CyA from 18 hpf, the nV (arrow), nVII (white arrowhead), and nX neurons (black arrowhead; out of focus) are mostly unaffected. oto, otocyst. EXPRESSION / LABELING:
|
|
Regulation of gli1 expression requires Gli3, but not Gli1 or Gli2, function. All panels show lateral views of the hindbrain with anterior to the left. Embryos in A–E were obtained from crosses between dtrte370+/- heterozygotes, while those in F–P were obtained from crosses between yotty119+/- heterozygotes. Krox20 expression (red) in ptc1;krox20 double in situ panels identifies rhombomeres 3 and 5. (A) In 21 hpf uninjected or gli2 MO-injected wild-type embryos, ptc1 is expressed at all axial levels in the ventral hindbrain. (B) In an uninjected dtr mutant, ptc1 expression is significantly reduced. (C) In a gli2 MO-injected dtr mutant, ptc1 expression is completely lost in the hindbrain. (D) In a 21-hpf uninjected or gli2 MO-injected wild-type embryo, gli1 is expressed at all axial levels in the ventral half/two-thirds of the hindbrain. (E) Gli1 expression is unaffected in control or gli2 MO-injected dtr mutants. (F–H) Ptc1 expression is normal in uninjected or gli1 MO-injected wild-type embryos (F), is reduced significantly in an uninjected yot mutant (G), and is completely lost from the hindbrain in a gli1 MO-injected yot mutant (H). (I and J) Gli1 is expressed normally in the ventral hindbrain in uninjected or gli1 MO-injected wild-type (I) or yot mutants (J). (K–M) Ptc1 expression is slightly reduced in a gli3 MO-injected 22 hpf wild-type embryo (K), is reduced significantly in an uninjected 24 hpf yot mutant (L), and is almost completely lost in a gli3 MO-injected 22 hpf yot mutant (M). (N–P) Gli1 is expressed normally in the ventral hindbrain in uninjected wild-type or yot mutant embryos (N), and in a gli3 MO-injected wild-type embryo (O). In a gli3 MO-injected yot mutant, gli1 expression is greatly reduced (P). EXPRESSION / LABELING:
|
|
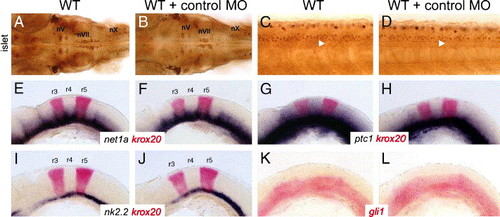
Control gli1 mismatch morpholinos have no effect on motor neuron development and expression of Hedgehog-regulated genes. Panels A, B, and C–L show dorsal and lateral views, respectively, with anterior to the left. (A and B) In a 36-hpf wild-type embryo, either uninjected (A) or control-morpholino injected (B), the islet antibody-labeled nV, nVII, and nX motor neurons are found in their characteristic positions in the hindbrain. The nX neurons are out of focus in both panels. (C and D) In a 36-hpf wild-type background, islet antibody-labeled spinal motor neurons (arrowheads) are found in similar numbers in both uninjected (C) and control morpholino-injected (D) embryos. (E–L) In uninjected 22 hpf wild-type embryos, Hedgehog-regulated genes such as net1a (E), ptc1 (G), nk2.2 (I), and gli1 (K) are expressed in characteristic patterns in the ventral hindbrain. In wild-type embryos injected with control morpholino, the expression patterns of net1a (F), ptc1 (H), nk2.2 (J), and gli1 (L) are not affected. Expression of krox20 in rhombomeres 3 and 5 was essentially the same in uninjected embryos (E, G, I) and control morpholino-injected embryos (F, H, J). |
Reprinted from Developmental Biology, 282(2), Vanderlaan, G., Tyurina, O.V., Karlstrom, R.O., and Chandrasekhar, A., Gli function is essential for motor neuron induction in zebrafish, 550-570, Copyright (2005) with permission from Elsevier. Full text @ Dev. Biol.