- Title
-
Regulation of the lmo2 promoter during hematopoietic and vascular development in zebrafish
- Authors
- Zhu, H., Traver, D., Davidson, A.J., Dibiase, A., Thisse, C., Thisse, B., Nimer, S., and Zon, L.I.
- Source
- Full text @ Dev. Biol.
|
EGFP expression under the control of a 2.5-kb lmo2 promoter recapitulates endogenous lmo2 expression. (a) Constructs used to generate the lmo2 transgenic lines are shown. Construct I is a 5-kb genomic fragment derived from a PAC clone that contains exons 1 and 2 of lmo2. Constructs II and III are 2.5 kb of the 5-kb fragment ligated to the reporter genes EGFP-1 and DsRed2-1. (b) EGFP and lmo2 transcripts first appear in two lateral stripes of ventral mesoderm (black arrowheads) by the 1-somite stage as shown in A and B. Transcripts can been detected at the 5-somite stage (C, D). Anterior vascular precursors express EGFP and lmo2 at 5 somites (E, F). At 12 somites, the posterior mesoderm stripes migrate to form the ICM (G). By the 18-somite stage, most of the ICM has formed from the convergent morphogenesis of the stripes (H, I). At 24 hpf, circulating blood (white arrow) and most of the vasculature, including the cardinal vein, dorsal aorta, and branching intersomitic vessels (white arrowhead), are fluorescent (J), which replicate that of endogenous lmo2 expression in K. In line LGb, EGFP is downregulated over the next 24 h, but vascular expression persists (L); the aorta and vein are fluorescently labeled during the formation of the presumptive AGM at 36 hpf (inset and white arrow). Endogenous lmo2 is expressed at low levels in the trunk vessels at 36 hpf (black arrow) (M). EXPRESSION / LABELING:
|
|
The lmo2 transgenic lines facilitate visualization of hematopoietic and vascular systems in wild-type and mutant embryos. (A) In LR embryos, DsRed protein is initially detected at 20 hpf (inset); 2 dpf LR embryos labeling hematopoietic (white arrowheads) and endothelial cells (DofC: ducts of Cuvier). (B) The cranial vessels of a 3 dpf LR embryo visualized by confocal microscopy (BA, basilar artery; Ant, Anterior; Post, posterior; Do, dorsal; Vent, ventral). (C) Confocal section through the head and trunk of a 3 dpf LR; Tg(fli1:EGFP) embryo displaying green/red cranial vessels (arrow, V) and red erythrocytes (arrowhead, E). (D,E) LR; Tg(fli1:EGFP) embryos distinctly label hematopoietic (arrowhead) and endothelial cells (arrows) in 24 hpf and 3 dpf embryos. In LR; Tg(gata1:EGFP) transgenic embryos, green/red erythrocytes (arrowheads) circulate through vessels (arrows) labeled by DsRed as shown in 24 hpf and 3 dpf embryos (F and G, respectively). In the second panel, 24–36 hpf mutant embryos carry the Tg(lmo2:EGFP) transgene. (H) In the zebrafish cloche mutant, there is an absence of hematopoietic cells and virtually no endothelium; EGFP expression is restricted to the posterior ICM (arrow), where lmo2 expression is known to be preserved. (I) In moonshine, normal vasculature is present (arrow), but erythrocytes are absent. (J) In mindbomb, vascular malformations (arrow points to fusion between artery and vein) are identified when EGFP-labeled cells travel from aorta into vein. |
|
Transplantation of primitive progenitors into vlad tepes (vlt) recipients. (A) The EGFP+ population from 10- to 12-somite LGb; Tg(gata1:DsRed) transgenic embryos was isolated by FACS to 90% purity and transplanted into 48 hpf vlt embryos. (B) One day after transplantation, circulating donor-derived cells were identified by DsRed and EGFP fluorescence. By 3 days post transplantation, most circulating cells were DsRed+, suggesting that the EGFP+ donor progenitors had differentiated into Gata1+ erythrocytes (arrow). One month after transplantation, each of the 8 survivors carried approximately 10–200 DsRed+ circulating cells (arrows). At 1 month, we also observed EGFP expression in the trunk vasculature (arrowheads) in 2 of 8 recipients. A table summarizing the results is shown in C. |
|
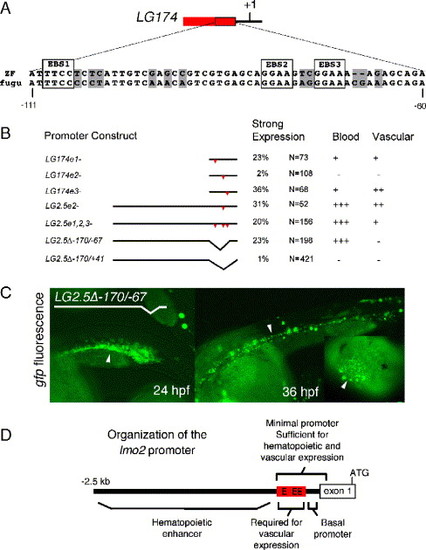
Deletion analysis identifies a compact promoter that drives hematopoietic and vascular expression. (A) The percentages of “strong expressers” (defined as >20 fluorescent cells in the ICM at 24–30 hpf) and relative levels of blood or vascular expression at 30 hpf are shown. (C) The developmental expression of the LG174 transgenic is shown at 5 somites, 18, 24, and 36 hpf. LG174 drives expression in the mesodermal stripe (ms, arrowhead), vascular cells (black arrows point to cv, cranial vessels; av, axial vessels), hematopoietic cells (white arrow, ICM), and trunk somites (black arrowhead, ts). |
|
Dissection of the lmo2 regulatory elements. (A) A 51-bp region from within the LG174 zebrafish promoter is 82% conserved with a 53-bp region in the fugu lmo2 locus. The three conserved ETS-factor binding sites (EBS) in this region are boxed. (B) Red triangles label EBS mutations. When EBS2 is mutated in LG174, strong expression is significantly decreased in ICM cells. When EBS1, EBS2, and EBS3 are mutated in the context of the entire 2.5-kb promoter, blood expression is normal, but endothelial expression is significantly reduced. When the region between -174 and -69 is removed from LG2.5 (called LG2.5Δ-170/-67), vascular expression is not seen, but blood expression is preserved. Removal of -174/+41 (LG2.5Δ-170/+41) resulted in no expression. (C) The transient expression pattern of LG2.5Δ-170/-67 at 24 and 36 hpf. At 24 hpf, the ICM is filled with EGFP+ cells (arrowhead) that are in circulation by 36 hpf. The inset shows blood cells (arrowhead) moving towards the heart at 36 hpf. No expression is seen in vascular endothelium at either stage. D shows the organization of lmo2 cis-elements. |
|
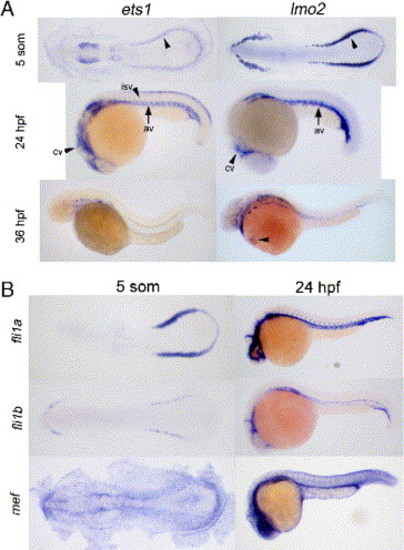
lmo2 is coexpressed with multiple ETS factors. (A) Developmental expression of ets1 and lmo2 at the 5-somite, 24 and 36 hpf stages. Both genes are expressed in the posterior mesoderm at 5 somites (arrowheads). At 24 hpf, both are expressed in the ICM, cranial (cv), and axial vessels (av). At 36 hpf, lmo2 is maintained in endothelial and circulating cells (arrowhead) while ets1 is in a subset of vessels but is not seen in circulating blood. (B) Other ETS factors are coexpressed with lmo2 in the ventral mesoderm at the 5-somite stage and ICM at 24 hpf. fli1a, fli1b, and mef/elf4 are ETS factors that could have redundant or cooperative roles with ets1 in regulating lmo2. EXPRESSION / LABELING:
|
|
ets1 and mef bind to the lmo2 promoter; ets1 activates the lmo2 promoter through EBS2. (A) EMSA of wild-type ets1 and C-terminal truncated ets1ΔN binding to oligo wtEBS1. Lane 1 contains labeled probe without lysate. Several nonspecific bands appear when labeled probe is incubated with unprogrammed lysate (lane 2). Neither version of ets1 can bind to labeled wtEBS1 (lanes 3 and 4). The second EMSA was performed with wild-type (wtEBS2,3) and mutant probes (e2-, e3-). Lane 1 contains labeled probe without lysate. A nonspecific band appears when labeled probe is incubated with unprogrammed lysate (lane 2). Wild-type ets1 exhibits minimal binding (lane 3), while ets1ΔN binds robustly (lane 4). ets1ΔN is used in lanes 4–10. Competition was performed with 50x and 500x excess of unlabeled wtEBS2,3 (lanes 5 and 6) as well as unlabeled mutant competitors (lanes 7–10). Lanes 7 and 8 show that the e2- mutant oligo is unable to compete away binding, which demonstrates that ets1ΔN specifically binds to EBS2. Alternatively, lanes 9 and 10 show that there is no specific binding to EBS3. The third EMSA shows mef binding to oligo wtEBS2,3 (lane 3). Lanes 4 and 5 show that mef specifically binds to EBS2 and lanes 6 and 7 show that binding to EBS3 can be competed off. (B) Shown in the first row of this panel are LGb embryos. ets1-injected LGb embryos exhibit a widened EGFP domain, diffuse paraxial mesoderm, and tailbud expression. In the second row, increased ICM as well as diffuse expression of EGFP are seen in injected LGb embryos at 22 hpf. In the third row, ets1 overexpression expands the lmo2 domain into the paraxial mesoderm. (C) Shown in the first row are LG174 stable transgenic embryos; within this group, ets1-injected embryos have higher levels of EGFP expression globally and locally in the stripes. ets1 activates EGFP expression in the yolk syncytium when co-injected with wild-type reporter construct LG174, but only has minimal activity when co-injected with LG174e2-, suggesting that ets1 activates the promoter through the binding of EBS2 in vivo. (D) Morpholino knockdown of ets1 (ets1 splice-MO) does not affect transgene expression. These morphant embryos have a severe reduction in ets1 as measured by whole mount in situ hybridization, demonstrating morpholino efficacy. EXPRESSION / LABELING:
|
Reprinted from Developmental Biology, 281(2), Zhu, H., Traver, D., Davidson, A.J., Dibiase, A., Thisse, C., Thisse, B., Nimer, S., and Zon, L.I., Regulation of the lmo2 promoter during hematopoietic and vascular development in zebrafish, 256-269, Copyright (2005) with permission from Elsevier. Full text @ Dev. Biol.