- Title
-
moz regulates Hox expression and pharyngeal segmental identity in zebrafish
- Authors
- Miller, C.T., Maves, L., and Kimmel, C.B.
- Source
- Full text @ Development
|
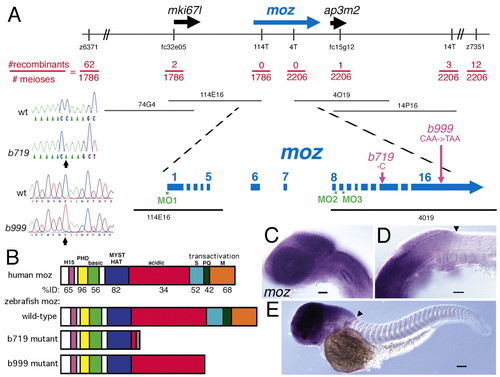
Mutations in a zebrafish moz. (A) Positional cloning of the gene disrupted by the b719 mutation. The LG5 genomic region is schematized at the top, with informative genetic markers shown. 4T, 14T and 114T are polymorphisms in the T7 end of PACs 4O19, 14P16 and 114E16, respectively. Position of four PACs are shown below the genomic region, with the moz region expanded underneath. moz spans the non-recombinant interval, with exons on both non-recombinant PAC ends. Lesions are schematized in purple and shown in the chromatograms on the left side of this panel: b719 deletes one bp (cytosine) in exon 14 of moz. A C-to-T missense mutation in b999 introduces an early stop codon in the 16th exon. Positions of morpholino oligos (MOs) are shown in green (see Tables 1 and 2). (B) Schematic of protein domains of human, zebrafish wild type, b719 mutant and b999 mutant. Amino acid domains (Kitabayashi et al., 2001a): H, H15 nuclear localization; PHD, PHD fingers; basic; MYST HAT, MYST family histone acetyltransferase; acidic; serine rich; glutamate rich; methionine rich. The percent identity between human and wild-type zebrafish is listed for each region beneath human MOZ. The C-terminal transactivation domain of human MOZ (Champagne et al., 2001; Kitabayashi et al., 2001a) is labeled. The gray domain in b719 mutant is frame-shifted prior to translation stop. (C-E) Embryonic expression of moz in wild types at 28 hpf (C,D) and 48 hpf (E). Lateral views of head (C), head/trunk interface (D) and whole larva (E). At both stages, moz expression appears ubiquitous in cranial tissues, and has a diffuse posterior border of expression near the boundary (arrowhead in D,E) of the hindbrain and spinal cord. Expression at these stages is not detected in the trunk and tail. Scale bars: 50 μm. |
|
Homeotic pharyngeal arch phenotype of moz mutant larvae. Pharyngeal cartilage phenotypes in wholemounts (A-C) and flat mounts (D-I) of wild-type (A,D,G), moz mutant (B,E,H) and moz-MO-injected (C,F,I) larvae at 4 days. (A-F) In moz mutants (E) and moz-MO-injected animals (F), both second arch cartilages (M′, pq′) adopt shapes resembling the first arch jaw cartilages. The HM region of HS is deleted (arrow in B). The transformation is incomplete in that the pterygoid process is not seen duplicated in moz mutants or moz-MO injected animals. An ectopic dorsal cartilage (arrowhead) is seen in the moz mutant third arch. (G-I) Third pharyngeal arch cartilages, with medial to the left. moz mutants (H) and moz-MO injected animals (I) display a homeotic third arch phenotype, with a process on their lateral end resembling the retroarticular process on Meckel′s cartilage. am, adductor mandibulae; ch, ceratohyal; hm, hyomandibular; hs, hyosymplectic; m, Meckel′s; pq, palatoquadrate; ptp, pterygoid process; sy, symplectic. Scale bars: 50 μm. PHENOTYPE:
|
|
moz is required for most hox1-4 expression domains. Lateral (A-D,G-H,M-V) and dorsal (E-F,I-L) views of Hox expression in wild type (A,C,E,G,I,K,M,O,Q,S,U) and moz mutants (B,D,F,H,J,L,N,P,R,T,V) at 33 hpf (A-F), 11 hpf (I,J) and 48 hpf (G,H,K-V). (A,B) hoxa2b expression, which is present in arches two and three of wild type (A), is undetectable in these arches in moz mutants (B). (C,D) hoxb2a expression normally present in the second arch (C), is barely detectable in the moz mutant second arch (D). (E,F) hoxa2b expression in the hindbrain is reduced in moz mutants (G,H) hoxa2b expression in second arch CNC is severely reduced (I,J) Initiation of hoxa2b expression at 11 hpf occurs normally in moz mutants. (K,L) hoxa1a in the ventral forebrain and midbrain, and anterior hindbrain, is unaffected in moz mutants (M-V) Graded affect on hoxb1a-b5a gene expression in moz mutants. (M,N) hoxb1a expression in r4 is largely abolished in moz mutants. (O,P) hoxb2a expression in r3-5 is downregulated in moz mutants, and second arch expression (arrow) is now undetectable. (Q,R) hoxb3a expression in the caudal hindbrain is reduced, and pharyngeal arch expression in arches 3-5 (arrow) is undetectable. (S,T) hoxb4a expression is mildly reduced in the posterior hindbrain and pharyngeal arches 4-6 (arrow). (U,V) hoxb5a expression appears unaffected in moz mutants. Scale bars: 50 μm. |
|
Rescue of skeletal homeosis and hoxa2b expression in moz mutants by the histone deacetylase inhibitor Trichostatin A (TSA). (A-D) Ventrolateral views of 4-day-old wild type (A,C) and moz mutant (B,D) larvae treated with DMSO (A,B) or TSA (C,D) stained with Alcian Green. TSA rescues many aspects of the skeletal phenotype, including deletion of the HM cartilage (arrowhead) and fusion and inversion of the ventral second arch cartilage (arrow; see Table 3). (E-H) Lateral views of hoxa2b expression at 33 hpf in wild type (E,G) and moz mutants (F,H) treated with DMSO (E,F) or TSA (G,H). TSA treatment rescues hoxa2b expression in moz mutants. e, eye. Scale bars: 50 μm. |
|
Neuronal patterning defects in moz mutants. (A-D) Cranial motoneurons labeled by Islet1:GFP (Higashijima et al., 2000) at 48 hpf in wild type (A,C) and moz mutants (B,D). (A,B) Most moz mutants (52%, 11 of 21) show defects in cell body positioning of facial motoneurons (arrow). (C,D) Cranial nerves innervate each pharyngeal arch in moz mutants. The VIIIth nerve octavolateralis efferent (arrowheads in C,D) is variably mispatterned in moz mutants, resembling the phenotype seen in hoxb1a-MO injected larvae (McClintock et al., 2002). |
|
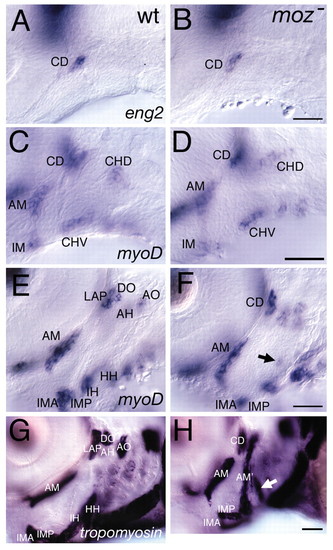
Late, but not early, muscle defects in moz mutants. (A,B) eng2 expression in wild types marks the dorsal first arch myogenic core, CD, which is not homeotically duplicated in moz mutants. (C-F) myod expression in wild type (C,E) and moz mutants (D,F) at 44 hpf (C,D) and 54 hpf (E,F). (C,D) myod expression marks three first arch and two second arch myogenic cores. The moz mutant second arch pattern at this stage appears normal. (E,F) By 54 hpf moz mutant musculature looks aberrant. A small ectopic patch of myod is present in the intermediate second arch (arrow in F). (G,H) Lateral views of α-tropomyosin expression in wild type (G) and moz mutant (H). In wild types (G), a large jaw closing muscle (am) connects the upper and lower jaw. moz mutants appear to have an ectopic jaw closer muscle (am′) in their second arch (H). This muscle appears continuous with what we interpret to be the remnants of the dorsal (AH and AO) and ventral muscles (IH and HH) and the first arch dorsal muscles (LAP and DO) appear to not have segregated as they have in wild types. An enlarged third arch muscle is present (white arrow in H) in moz mutants. AH, adductor hyomandibulae; AM, adductor mandibulae; AO, adductor operculi; DO, dilator operculi; HH, hyohyal; IH, interhyal; IMA, intermandibularis anterior; IMP, intermandibularis posterior; LAP, levator arcus palatini. Scale bars: 50 μm. |
|
Early patterning of arch epithelial tissues appears unaffected in moz mutants. Lateral views of wild-type (A,C,E,G) and moz mutant (B,D,F,H) embryos at 34 hpf (A,B), 41 hpf (C,D), 54 hpf (E,F) and 4 days (G,H). (A,B) pea3 expression marks posterior pharyngeal endodermal epithelia. No inversion of this pattern is seen in moz mutant pouches. First and second pharyngeal pouches are outlined. (C,D) sonic hedgehog (shh) expression marks the posterior ectodermal margin (PEM) of the second arch, a thin line of cells marking the posterior edge of the forming opercular flap. PEM expression persists in moz mutants. (E,F) pitx2c expression strongly labels the mouth in wild type and moz mutant. No ectopic pitx2c expression is seen in the mouth-like openings between the second and third arch in moz mutants. (G,H) rag1 expression labels a reduced but present thymus in moz mutants. pp1, pharyngeal pouch 1; pp2, pharyngeal pouch 2. Scale bars: 50 μm. |
|
Hyoid CNC is generated in moz mutants but forms mispositioned and misshapen condensations. Confocal micrographs of lateral views of the anterior pharyngeal arches in live wild-type (A,C,E,G) and moz mutant (B,D,F,H) embryos (A-F) and larvae (G-H) stained with the vital fluorescent dye BODIPY-ceramide at 28 hpf, 34 hpf, 48 hpf and 3.5 day stages. Images have been inverted; the dye fills interstitial spaces, so the inverted images show cells labeled in white and interstitial space in black. (A,B) Early CNC appears morphologically indistinguishable from wild type in moz mutants. Both first and second arches are filled with a cylinder of postmigratory CNC (Miller et al., 2000; Kimmel et al., 2001b), with no obvious defect in hyoid or branchial CNC in mutants. Other arch tissues also fail to show obvious morphological defects (C,D). At a slightly later stage the second pharyngeal arch in moz mutants appears slightly hypoplastic (D), but is still filled with CNC. (E,F) A day later, condensations have formed in the wild type (E), including in the second arch a dorsal hyomandibular condensation (arrow, compare with Fig. 2D) and a ventral ceratohyal condensation (arrowhead). In moz mutants, no dorsal second arch hyomandibular condensation is seen and cells appear as lose mesenchyme in this region (arrow in F). A dorsal mutant condensation does form, in the position of the wild-type symplectic condensation. The ventral mutant condensation is mispositioned anteriorly (arrowhead), and is abutting the first arch ventral condensation. The condensation pattern (E,F) largely prefigures the resultant larval cartilage pattern (G,H; compare with Fig. 2D,E). This figure shows two time points of four different animals: A and C are the same animal, as are B and D, E and G, and F and H. Arches are numbered in A-D. |
|
Stable ectopic expression of bapx1 in the moz mutant second arch. Ventral (A-C,F-I) and lateral (D,E) views of bapx1 expression in whole-mount wild-type (A,D,F,H), moz mutant (B,E,G,I) and hoxa2b + hoxb2a-MO co-injected animals (C) at 33 hpf (A-E), 54 hpf (F,G) and 4 days (H,I). (A-E) bapx1 expression, which is normally restricted to a patch of first arch mesenchyme (A,D), is ectopically expressed (arrowhead in E) in the second arch of moz mutants (B,E) and hoxa2b+hoxb2a-MO co-injected animals (C). (F-I) Ectopic bapx1 expression (arrowheads) is maintained in second arch mesenchyme of moz mutants. (J,K) Reduction of bapx1 function in wild type (J) and moz mutant (K). Reducing bapx1 function in wild type causes specific loss of the jaw joint (asterisk in J), while reducing function of bapx1 in moz mutants causes loss of both first and second arch joints (asterisks in K). Reduction of bapx1 function in moz mutants also can rescue the ventral arch one and two fusions. The first two pharyngeal arches are numbered. e, eye. Scale bars: 50 μm. |
|
Mirror-image duplication of gsc expression in the moz mutant second arch. Ventral (A-C,E,G) and lateral (D,F) views of gsc expression in whole-mount wild-type (A,D,E), moz mutants (B,F,G) and hoxa2b+hoxb2a-MO co-injected animals (C) at 33 hpf (A-C) and 41 hpf (D-G). In moz mutants and hoxa2b+hoxb2a-MO co-injected animals, second arch expression shift medially (arrows in B and C), mirroring the wild-type first arch pattern. (D-G) At 41 hpf in moz mutants, dorsal second arch gsc expression is reduced (arrowhead in F) and ventral expression is still inverted (arrows in E,G). (H) Schematic of expression domains visible in (A,B,E,G). In moz mutants, lateral second arch gsc expression is missing, and instead the second arch pattern resembles a mirror-image duplication of the first arch pattern, with a thin medial crescent of expression in the anterior medial arch. Relevant pharyngeal arches are numbered. e, eye. Scale bars: 50 μm. |