- Title
-
The Netrin receptor Neogenin is required for neural tube formation and somitogenesis in zebrafish
- Authors
- Mawdsley, D.J., Cooper, H.M., Hogan, B.M., Cody, S.H., Lieschke, G.J., and Heath, J.K.
- Source
- Full text @ Dev. Biol.
|
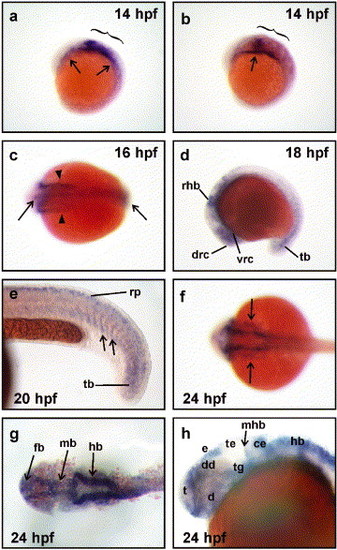
Expression of neogenin during zebrafish development. (a and b) neogenin mRNA expression was first detected by whole mount in situ hybridization at 14 hpf in the presumptive hindbrain (brackets) and in the lateral plate mesoderm (arrows). (c) At the onset of neurulation (16 hpf), neogenin expression was observed throughout the rostrocaudal extent of the neural rod (arrows) as well as in the lateral plate mesoderm (arrowheads). (d) At 18 hpf, neogenin expression localized to regions of the forebrain, midbrain, and hindbrain, including areas encompassing early clusters of differentiating neurons, for example, the dorsorostral cluster (drc) in the telencephalon and the ventrorostral cluster (vrc) in the rostral diencephalon. Expression was also detected in the rostral hindbrain (rhb) and tailbud (tb). (e) At 20 hpf, neogenin expression was found in the ventral portion of the most recently formed somites (arrows), tailbud, and the roof plate (rp) within the neural tube. (f-h) At 24 hpf, expression was observed in the lateral mesoderm (f, arrows) and (g) the neuroepithelium of the forebrain (fb), midbrain (mb), and hindbrain (hb). (h) neogenin expression was detected in neuronal clusters of the forebrain including in the telencephalon (t), diencephalon (d), including its dorsal region (dd), and the epiphysis (e). Meanwhile, midbrain expression was strong in the tegmentum (tg) but negligible in the tectum (te) and the midbrain component of the midbrain-hindbrain boundary (mhb). Strong expression was seen in the cerebellum (ce) and dorsal hindbrain. (a, b, d, e, and h) Lateral views with rostral to the left and dorsal to the top. (c, f, and g) Dorsal views with rostral to the left. EXPRESSION / LABELING:
|
|
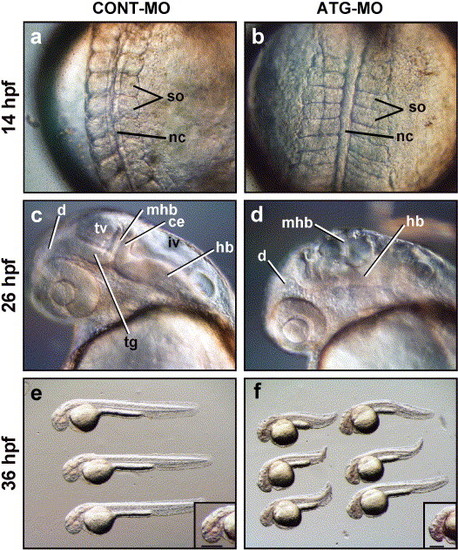
The development of the CNS and somites is impaired in Neogenin knockdown embryos (morphants). Neogenin morphants (b, d, and f) were generated using a neogenin antisense morpholino oligonucleotide (ATG-MO; 3.7 ng). Controls (a, c, and e) were generated using a control morpholino oligonucleotide (CONT-MO; 3.7 ng). (a) Normal somite (so) development at the 10 somite stage (14 hpf) in embryos injected with CONT-MO. (b) Somites of the ATG-MO-injected embryos were thinner along the rostrocaudal axis and wider along the mediolateral axis. (c) At 24 hpf, key structures of the early CNS such as the midbrain-hindbrain boundary (mhb), tectal ventricle (tv), fourth ventricle (iv), tegmentum (tg), and cerebellum (ce) were precisely delineated in embryos injected with CONT-MO while (d) ATG-MO-injected embryos exhibited severe perturbations in the development of these CNS structures. (e) 36 hpf embryos injected with CONT-MO showed no gross morphological abnormalities. (f) By 36 hpf, the rostrocaudal body axis of the morphants was shorter than that of control embryos. The length of the dorsoventral axis of the developing brain was reduced in morphants, indicated by the reduced length of the horizontal bar (inset) compared to controls, suggesting a loss of neural tissue. d, Diencephalon; hb, hindbrain; nc, notochord. (a and b) Dorsal views with rostral to the top. (c-f) Lateral views with rostral to the left and dorsal to the top. PHENOTYPE:
|
|
Nonoverlapping morpholinos targeted to neogenin mRNA produced indistinguishable morphant phenotypes. (a-i) Reduction in the number of post-mitotic neurons in Neogenin morphants. Whole mount immunohistochemistry was used to visualize post-mitotic neurons at 24 hpf in embryos injected with the antisense morpholinos (ATG-MO and UTR-MO) or control morpholino (CONT-MO). (a, d, and g) Embryos injected with 4 ng CONT-MO. (b, e, and h) Embryos injected with 4 ng of ATG-MO. (c, f, and i) Embryos injected with 4 ng of the UTR-MO. (a) The control embryos displayed a morphologically normal and readily discernable spinal cord (sc), floorplate (fp), notochord (no), and V-shaped somites (so). (b and c) The spinal cord, floorplate, and notochord of the ATG-MO and UTR-MO Neogenin morphants were not as regular or well-defined as in the controls, and the somites were U-shaped. (d and g) At 24 hpf, a continuous population of Hu-positive post-mitotic neurons existed along the rostrocaudal axis of the spinal cord of the control embryos. (e, f, h, and i) The ATG-MO and UTR-MO Neogenin morphants displayed a reduced number of Hu-positive neurons with prominent gaps along the rostrocaudal axis of the spinal cord (arrows). (j-l) Marked increase in the number of dead cells in the neuroepithelium of Neogenin morphants. Embryos (24 hpf) were stained with acridine orange (AO) to indicate cell death. The embryos were flat-mounted for imaging in the confocal microscope. Main picture is a maximum intensity projection of the z series; inset shows a single confocal section to demonstrate that the dead cells are in the same plane as the neuroepithelium. There was a marked increase in the number of AO-positive cells present in the CNS in embryos injected with 5 ng of either the ATG-MO or the UTR-MO (j and k) compared to embryos injected with 5 ng CONT-MO (l). (j-l) Dorsal views with rostral to the top. PHENOTYPE:
|
|
Severe reduction in number and density of axon tracts in Neogenin morphants. Whole-mount immunohistochemistry using an anti-α-acetylated tubulin monoclonal antibody was used to visualize the major axon tracts in 24 hpf embryos. (a) In control embryos (3.7 ng CONT-MO), all major axon tracts were present including the dorsal longitudinal fasciculus (dlf), the ventral longitudinal fasciculus (vlf) of the developing neural tube, the medial lateral fasciculus (mlf) coursing from the hindbrain, as well as the anterior (ac), posterior (pc), and post-optic commissures (poc). Axons emanating from the trigeminal ganglia (tgg) and the caudal primary (CaP) motor neurons, which project from the ventral neural tube to the ventral myotome, were readily observed. (b-d) In morphant embryos of varying phenotypic severities (b-d; 7.5, 3.7, and 2.5 ng ATG-MO, respectively), a severe reduction in the number and density of axon tracts along the entire rostrocaudal axis of the embryo was observed. The intense staining in the forebrain, the midbrain, and hindbrain seen in the control brain was almost completely lost. In addition, the mlf, dlf, and vlf in the neural tube comprised significantly fewer fibers. Axons from CaP neurons were also largely absent. (a-d) Lateral views with rostral to the left and dorsal to the top. PHENOTYPE:
|
|
Loss of differentiated neuronal populations in Neogenin morphants. (a-d) Compared to CONT-MO (3.7 ng)-injected embryos at 24 hpf (a), ATG-MO (3.7 ng)-injected embryos (b) displayed a reduction in isl-1 expression in primary motor neurons (mot) and Rohon-Beard sensory neurons (RB) along the entire length of the neural tube. In the brain primordia of morphants (d), isl-1-expressing neurons were also reduced in number including those in the dorsorostral cluster (drc) of the telencephalon, the trigeminal ganglia (tgg), and the reticulospinal neurons of the hindbrain (rsn) compared to control embryos (c). (f) A proportion of pax2.1-expressing neurons was absent from the hindbrain of ATG-MO-injected embryos (arrows) compared to control embryos (e). (g) In control embryos, hlx-1 was expressed by populations of neurons in the tegmentum (tg), tectum (te), diencephalon (d), hindbrain (hb), and neural tube. (h) Neogenin morphants exhibited a similar pattern of hlx-1-expressing cells in the dorsal region of the diencephalon (d), tectum, and tegmentum. (a-d, g, and h). Lateral views with rostral to the left. (e and f) Dorsal views with rostral to the left. mhb, midbrain-hindbrain boundary; op, otic placode. |
|
Neural progenitor populations are specified and positioned correctly in Neogenin morphants. (a) shh was expressed in the diencephalon (d) and floor plate (fp) within the developing brain and neural tube in control embryos (26 hpf). (b) The overall pattern of shh expression remained unaltered in the embryos injected with 3.7 ng ATG-MO, except for the dorsally directed peak of expression in the caudal diencephalon, which was reduced. (c) Expression of pax2.1 delineated the rostral domain of the midbrain-hindbrain boundary (mhb) in control embryos (26 hpf). (d) The pattern of pax2.1 expression was identical in the morphants indicating that mhb patterning was unaffected. (e) The expression of krox-20 in control embryos delineated rhombomeres 3 and 5 in the developing hindbrain (20 hpf). (f) The expression of krox-20 in the morphants was indistinguishable from control embryos. (a-f) Lateral views with rostral to the left and dorsal to the top. EXPRESSION / LABELING:
|
|
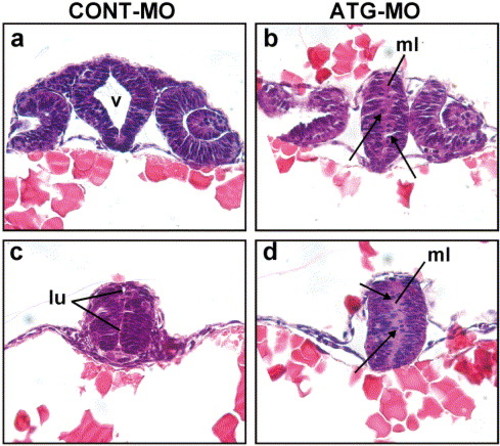
Failure to form a lumen in the neural tube of Neogenin morphants. (a) Oblique transverse section through the telencephalon towards the ventral diencephalon stained with hematoxylin and eosin demonstrated that the diencephalic ventricle (v) was formed in the control by 24 hpf. (b) Equivalent section from a Neogenin morphant where no ventricle was formed. (c) The lumen (lu) of the rostral neural tube was apparent in control embryos at 24 hpf. (d) The lumen was absent in the rostral neural tube of morphants. Cells in the bilateral columns of neuroepithelium were fewer and poorly organized in Neogenin morphants (b and d) compared to those in control embryos (a and c). Some cells had delaminated from the neuroepithelium in the morphants and were ectopically positioned in the region where the lumen would normally have formed (b and d: arrows). ml, midline. PHENOTYPE:
|
|
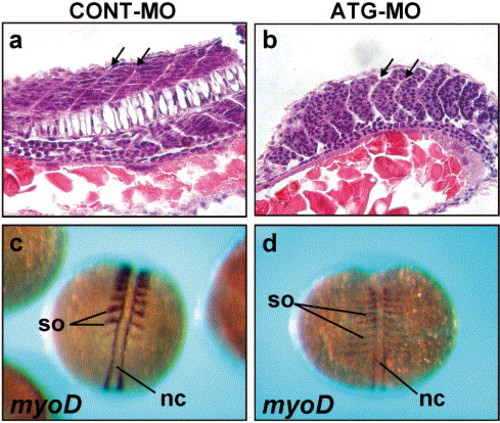
Aberrant somitogenesis in Neogenin morphants. (a-b) Sagittal sections through the trunk of 24 hpf embryos stained with hematoxylin and eosin. (a) Within the somites of control embryos, cells lined up to form discrete V-shaped epithelial boundaries (arrows) surrounding more loosely packed mesenchymal cells in the interior. (b) In the morphants, U-shaped epithelial boundaries (arrows) were formed and boundary cells remained rounded and loosely packed. Fewer mesenchymal cells were present within the interior of each somite. (c) At 14 hpf (10 somites), myoD expression was present in the caudal half of each somite (so) and the adaxial cells situated adjacent to the notochord (nc) in control embryos. (d) Morphant embryos exhibited the expected myoD expression pattern; however, the width of myoD expression in each somite was markedly reduced in the rostrocaudal direction and expanded in the mediolateral direction. (a and b) Rostral to the left, dorsal to the top. (c and d) Dorsal views with rostral to the top. PHENOTYPE:
|

Unillustrated author statements |
Reprinted from Developmental Biology, 269(1), Mawdsley, D.J., Cooper, H.M., Hogan, B.M., Cody, S.H., Lieschke, G.J., and Heath, J.K., The Netrin receptor Neogenin is required for neural tube formation and somitogenesis in zebrafish, 302-315, Copyright (2004) with permission from Elsevier. Full text @ Dev. Biol.