- Title
-
Sequential antagonism of early and late Wnt-signaling by zebrafish colgate promotes dorsal and anterior fates
- Authors
- Nambiar, R.M., and Henion, P.D.
- Source
- Full text @ Dev. Biol.
|
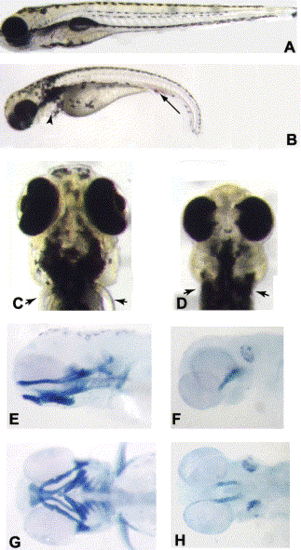
(A,B) Wild-type (A) and col mutant (B) embryos at 3.5 dpf. col mutant embryos have a shorter and curved body axis and also display an accumulation of blood cells (arrow in B) and edema of the heart (arrowhead). (C,D) Ventral views of the head of a wild-type (C) and col (D) mutant embryo at 3.5 dpf. col embryos have a smaller head with reduced, partially cyclopic eyes. Arrowheads mark the pectoral fins in wild-type (C) embryos. col mutants lack pectoral fins (arrowheads in D). (E–H) Alcian blue preparations of 3.5 dpf embryos to reveal craniofacial cartilages. Lateral (E,F) and ventral (G,H) views of wild-type (E,G) and col mutant (F,H) embryos illustrate the dramatic reduction of cartilages in mutant embryos. |
|
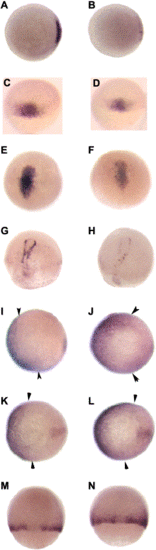
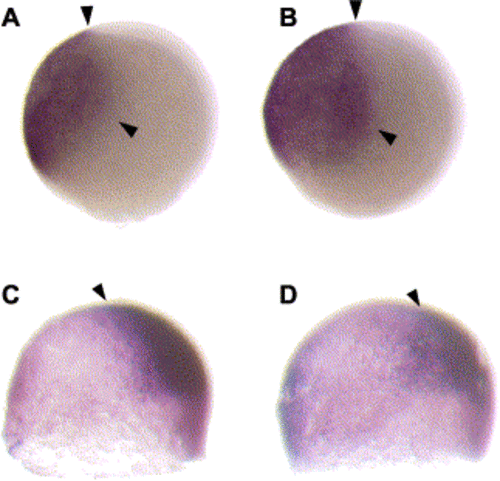
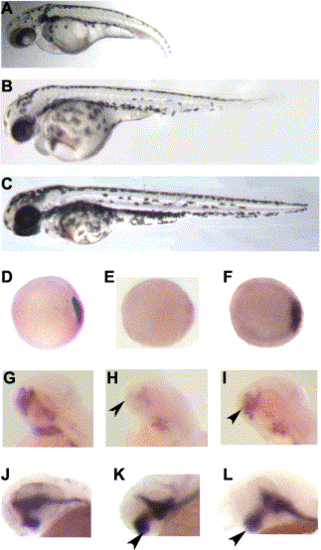
col mutants display a reduction in the expression of shield-specific markers (A–D). Animal pole views of 6 hpf (shield stage) wild-type (A, C) and col mutant (B,D) embryos. gsc is normally expressed in the shield of wild-type embryos (A) and gsc expression is down-regulated in col mutant embryos (B). A mild reduction is also seen in chd expression in the shield at 6 hpf in col mutants (D) as compared to wild-type embryos (C). A decrease in expression of prechordal plate markers is also seen in col mutant embryos (E–H). At 80% epiboly (8.5 hpf), gsc is expressed in the developing prechordal plate of wild-type embryos (E). Prechordal plate gsc expression is reduced in col mutants (F). A similar decrease in expression is seen in dorsal views of col mutant embryos at 90% epiboly (H), when dkk1 expression is observed in the prechordal plate in wild-type embryos (G). col embryos display an expansion in the expression of ventralizing signals. (I–L) Animal pole views of col mutant embryos at 80% epiboly show an expansion of bmp2 (J) and bmp4 expression (L) as compared to wild-type embryos (I,K). Arrowheads indicate approximate limits of expression domains. (M,N) In dorsal views, wnt8 expression is apparent in the ventrolateral mesoderm and is excluded from the dorsal midline in wild-type embryos (M) at 60% epiboly (7 hpf). In col mutant embryos (N) at the same stage of development, wnt8 expression expands into the dorsal midline. |
|
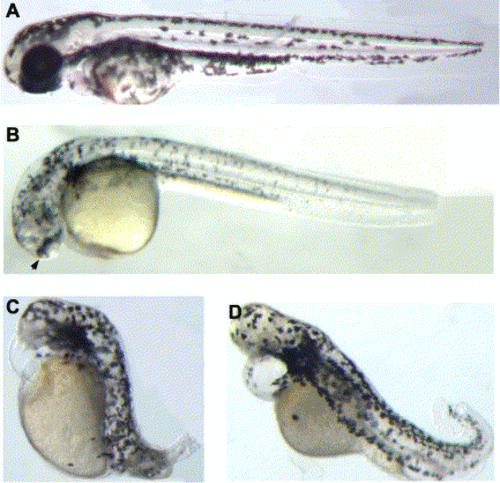
col mutant embryos display an increase ventral and posterior mesoderm-derived cells including blood and tail bud cells as well as a decrease in dorso-anterior mesoderm-derived cardiac mesoderm. (A–D) gata1 is expressed by hematopoietic precursor cells of 10- (A) and 26- (C) somite-stage wild-type embryos. At the same developmental stages, there are increased numbers of gata1-expressing cells in col mutants (B,D). The arrowhead in D marks the large number of blood cells in the region of the anus in col embryos. (E,F) At 60% epiboly (7 hpf), ntl is expressed in the ventrolateral mesoderm along the blastoderm margin of wild-type embryos (E). ntl expression is expanded in col mutant embryos (F). ntl at the 10-somite stage (G,H) and wnt8 at the 3-somite stage (I,J) are expressed by mesodermal cells of the tail bud. Increased expression of both ntl (H) and wnt8 (J) in the tail bud mesoderm is observed in col mutant embryos. Twenty-six-somite- (K) and 16-somite- (M) stage, wild-type embryos showing apoptotic cells revealed by TUNEL. The increase in the number of tail bud cells in col mutants is accompanied by an increase in the number of apoptotic cells in the tail (L). The arrow marks the TUNEL -positive cells in the tail of col mutants. (M,N) Lateral views of wild-type (M) and col mutant (N) embryos processed for TUNEL with arrow (N) indicating apoptotic cells ventral to the forming yolk extension in mutant embryos. (O,P) Lateral views of cardiac mesoderm nkx2.5 expression in 24 hpf wild-type (O) and col mutant (P) embryos reveal a reduction in the expression domain in col mutant embryos. (Q,R) dHAND expression in the developing heart (arrows) in 48 hpf wild-type (Q) and col mutant embryos (R) reveals decreased expression in col mutant embryos. |
|
col mutants display an expansion of non-neural ectoderm and a reduction in neural tissue during late gastrulation. (A,B) At 80% epiboly (8.5 hpf; A; lateral view), gata2 is expressed by non-neural ectoderm in wild-type embryos. The domain of gata2 expression is expanded dorsally and anteriorly in col mutants (B). Arrowheads in A and B denote limits of the gata2 expression domains. (C,D) At 75% epiboly (8 hpf; C; lateral view), otx2 is expressed by the forebrain and midbrain anlage of the neuroectoderm in wild-type embryos (C). In col mutants (D), otx2 expression is reduced and the anterior border of the expression domain (arrowheads) does not reach the animal pole. |
|
col mutants display regionalized posteriorization within the anterior neuroectoderm. (A,B) anf is expressed along the anterior edge of the neuroectoderm in wild-type embryos (A; dorsal view) at tailbud stage and anf expression is decreased in col mutant embryos (B). (C–F) The telencephalic stripe of dlx2 expression in a lateral view of a (C,D) 24 hpf and (E,F) 40 hpf col mutant (D,F arrowhead) is reduced compared to wild-type (C,E). (G,H) Lateral views of ventral diencephalic expression of shh in 32 hpf wild-type (G) and col mutant (H) embryos illustrates the anterior expansion of shh expression (arrowhead in H) in col mutant embryos. (I,J) At 90% epiboly, wnt8b is expressed at the MHB in wild-type embryos (I). wnt8b expression is expanded in col mutants (J). (K,L) The number of tyrosine hydroxylase-expressing dopaminergic neurons of the midbrain is reduced in col mutant (L) embryos as compared to wild-type (K) embryos at 3 dpf. (M,N) pax2.1 expression at the MHB in 48 hpf wild-type (M) and col mutant (N) embryos. Arrowheads indicate the expanded anterior–posterior extent of pax2.1 expression in col mutant embryos. |
|
col mutant embryos display a reduction of neural tissue and regional posteriorization of the neuroectoderm. (A,B) Dorsal views of wild-type (A) and col mutant (B) embryos at 2 dpf labeled with zn5 antibody. The developing brain in col mutants appears laterally reduced (B). (C,D) Ventral views of the heads of wild-type (C) and col mutant (D) embryos at 2 dpf labeled with anti-acetylated tubulin antibody with arrowheads indicating the olfactory organs that are reduced in size in col mutants. (E,F) Lateral views of wild type (E) and col mutants (F) labeled with anti-acetylated tubulin antibody at 2 dpf. Arrow in (E) marks staining in the tectum of wild-type embryos. A dramatic reduction in staining in seen in col mutants (F). (G,H) Dorsal views of wild-type (G) and col mutant (H) embryos at 48 hpf labeled with zn12 antibody with arrowheads marking the anterior–posterior extent of the tectum. The size of the tectum is reduced in col mutants whereas the cerebellum is larger (arrow in H). (I,J) A drastic reduction in tectal projections is evident in 2 dpf col mutants (J) labeled with zn5 antibody as compared to wild type (I). |
|
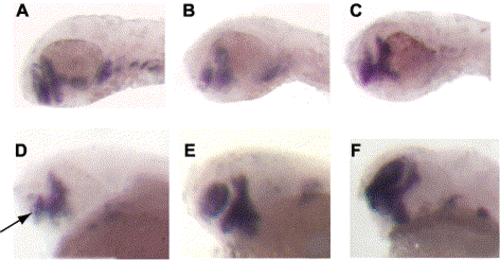
Expression of Wnt pathway antagonists partially rescue the col mutant phenotype. (A–C) The telencephalic stripe of dlx2 expression is reduced in col mutants (B) as compared to wild-type embryos (A) at 48 hpf. dlx2 expression is restored in col mutants injected with gsk-3β mRNA (C). Telencephalic expression of dlx2 is restored in col embryos injected with dkk1 mRNA (E,F) compared to uninjected col mutants (D) at 40 hpf. |
|
Antisense morpholino-mediated knockdown of wnt8 rescues the shield and forebrain phenotypes of col mutant phenotype. (A–I) An uninjected col mutant embryo at 3 dpf (A), a col mutant embryo injected with wnt8 MO (B) and an uninjected wild-type embryo (C) at 3 dpf. gsc expression in wild-type embryos (D) at shield stage (6 hpf) is reduced in col mutants (E, see Figs. 3A,B). gsc expression is restored in col mutants injected with wnt8 MO (F). dlx2 expression in the telencephalon (arrowhead) of injected col embryos at 48 hpf is rescued (I) as compared to uninjected mutants (H) and uninjected wild-type embryos (G). Expanded diencephalon revealed by shh expression (arrows) still persists in injected col mutants embryos at 40 hpf (L) as in uninjected mutant embryos (K) and unlike uninjected wild-type embryos (J). |
|
Antisense morpholino-mediated knockdown of wnt8b rescues regional patterning defects in the brain of col embryos. (A–I) The midbrain–hindbrain boundary region marked by pax2 expression is expanded in col mutants (B) as compared to wild-type embryos at 40 dpf (A). Injection of wnt8b MO results in a reduction in MHB tissue in mutant embryos (C). The extent of the MHB region is shown with arrowheads. wnt8b MO injection of mutant embryos abolishes ectopic diencephalic shh expression (F) observed in uninjected col embryos at 32 hpf (E, arrow; see Figs. 8G,H). Injected mutant embryos (F) strongly resemble uninjected wild-type embryos (D). The reduced telencephalic dlx2 expression in uninjected col embryos (H; see Figs. 8E,F) is rescued in injected embryos (I, compare to wild type, G). The telencephalic stripe of dlx2 expression is marked with arrows. Early patterning events during gastrulation are unaffected in wnt8b morpholino-injected col mutants (J–O). gsc expression is down-regulated in the prechordal plate in uninjected (K) and injected (L) col embryos as compared to wild-type embryos at 70% epiboly (J). wnt8 expression is ectopically expressed in the dorsal midline in uninjected (N) and injected (O) col mutant embryos unlike in wild-type embryos (M) at 70% epiboly. |
|
Expression of dominant-negative forms of negative regulators of Wnt signalling enhances the col mutant phenotype. (A–D) Injection of dominant-negative Xgsk-3β results in a partial loss of anterior tissue and reduced eyes in wild-type embryos (B) compared to uninjected wild-type embryos (A). The arrowhead marks a reduced, cyclopic eye in an injected wild-type embryo. Expression of dominant-negative axin (GID-2 fragment; C) and dominant-negative Xgsk-3β (D) in col mutant embryos results in a complete loss of eyes and anterior tissue. All embryos are 48 hpf. |
Reprinted from Developmental Biology, 267(1), Nambiar, R.M., and Henion, P.D., Sequential antagonism of early and late Wnt-signaling by zebrafish colgate promotes dorsal and anterior fates, 165-80, Copyright (2004) with permission from Elsevier. Full text @ Dev. Biol.