- Title
-
Interplay between FGF, one-eyed pinhead, and T-box transcription factors during zebrafish posterior development
- Authors
- Griffin, K.J. and Kimelman, D.
- Source
- Full text @ Dev. Biol.
|
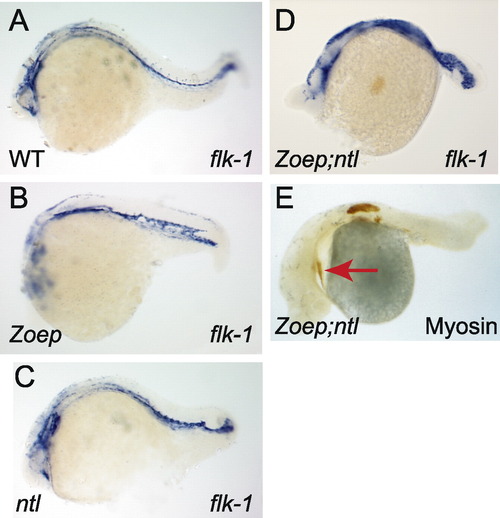
Zoep and ntl are not required for the formation of cardiac mesoderm or vascular endothelium. Anterior, left; dorsal, uppermost; genotypes as indicated (bottom left) (A–D) flk-1 expression. Note the presence of flk-1 expressing cells in the Zoep;ntl embryo, which are disorganized and possibly more numerous than in wild-type or either single mutant embryos. (E) Myosin staining (brown) detects the cardiac primordium (arrow), as well as somitic tissue in the anterior trunk, as previously reported. |
|
Zoep and ntl are together required to maintain spt expression. (A–D) seven hours (60% epiboly), dorsal view, animal pole uppermost. (E and F) seven hours (60% epiboly), vegetal view. (G–I) Ten hours (bud stage), posterior view of the tail bud, dorsal is up. (A) Wild-type embryo. Spt is expressed in paraxial mesoderm progenitors involuting at the margin and the migrating prechordal mesoderm (arrow), but is excluded from the notochord progenitors (N). (B) Ntl mutant embryo; spt is expressed in paraxial and prechordal mesoderm (arrow), but is weaker in paraxial mesoderm adjacent to the notochord progenitors and the border of spt expression lacks the defined edge observed in wild-type embryos. (C) Zoep mutant embryo; spt expression is normal in cells at the margin, but is not observed in prechordal mesoderm progenitors. (D) Zoep;ntl double mutant embryo; spt is expressed at the margin but is weaker adjacent to the notochord and is not detected in prechordal mesoderm progenitors. (E and F) Vegetal view, dorsal side uppermost, of embryos in C and D, showing the distribution of spt expression in mesoderm at the margin. (G) Expression of spt in a wild-type embryo. Note the intense staining in the tail bud (arrow) and segmental plate cells either side of the nonexpressing notochord. (H) Zoep;ntl double mutant embryo; spt expression is only observed weakly in a small number of cells in the tail bud (arrow). (I) cyc;sqt double mutant embryo. Note the intense expression of spt in the tail bud. |
|
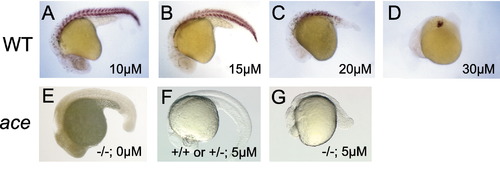
Effect of the FGFR inhibitor SU5402 on posterior development in wild-type and ace/fgf8 mutant embryos. (A–D and F) Wild-type and (E and G) ace mutant embryos. All embryos are 24–32 h, anterior to the left. Embryos are stained with an antibody to detect myosin, except E–G, which are unstained. (A–D) Increasing the concentration of SU5402 led to a dose-dependent loss in muscle staining, beginning with the tail. Only tail muscle defects were observed at 15 μM, whereas trunk and tail muscle defects were observed at 30 μM. (E–G) Embryos from obtained from ace/+ adults. (E) Untreated ace mutant embryo, showing typically good posterior development. (F) Wild-type embryo from ace/+ parents treated with 5 μM Su5402; (G) ace mutant embryo treated with 5 μM SU5402 showing severe posterior defects. |
|
Spt and ntl mutant embryos are hypersensitive to FGFR inhibition. (A–C) Embryos from ntl/+ adults. (A) Myosin staining in an untreated ntl mutant embryo. (B) Presumptive ntl mutant embryo treated with 4 μM SU5402; myosin staining is dramatically reduced relative to (A) and (C) ntl +/+ and +/- embryos treated with 4 μM SU5402. (D–F) Embryos obtained from spt/+ adults. (D) Untreated spt mutant embryos have patchy myosin staining in the trunk and segmented staining in the tail. (E) spt mutant embryos treated with 7 μM SU5402 are almost devoid of myosin staining, whereas (F) spt +/+ and +/- embryos treated with 7 μM SU5402 appear similar to untreated wild-type embryos. |
|
The Zoep mutant phenotype is enhanced by FGFR inhibition. Embryos at 24 h of development, anterior to left, hybridized to detect myoD expression (brown stain). (A–C) Zoep +/?; (D–F) Zoep -/-. Defects in wild-type tail somitic mesoderm are first observed at 15 μM (C), as described in Fig. 3. (D) Untreated Zoep mutant embryo. (E) Zoep mutant embryos treated with 10 μM show aberrations in the number of myoD-positive cells. Unlike the defects observed in wild-type embryos treated with 10 μM SU5402, loss of muscle did not occur strictly from posterior to anterior, as indicated by the gap in myoD expression in the posterior trunk. Muscle staining in the tail was continuous across the midline, indicating the absence of posterior notochord. (F) Zoep mutant embryos treated with 15 μM; myoD staining is only detected in the anterior trunk. |
|
Zoep genetically interacts with ace/fgf8. All embryos are shown in lateral view, anterior to the left. (A, E, I, and M) wild-type; (B, F, and J) ace mutants; (C, G, and K) Zoep mutants; (D, H, L, and N) Zoep;ace double mutants. (A–D) Live embryos at 48 h. (A) Wild-type embryo. (B) ace mutant embryos lack the cerebellum and have an enlarged tectum (arrow). (C) Zoep mutant embryos are obviously cyclopic (arrow). Both ace and Zoep single mutants have well developed posterior mesoderm and neuroectoderm. (D) Zoep;ace double mutant embryo; the phenotype was additive in anterior neuroectoderm. Posterior development in Zoep;ace embryos was extremely poor, and mesodermal derivatives (somites and notochord) were difficult to distinguish morphologically. (E–H) MyoD expression (24 h). (E) Wild type, (F) ace, and (G) Zoep mutant embryos show strong myoD expression throughout the somites. (H) Severely affected Zoep;ace embryo; myoD is expressed only in a few cells in the anterior trunk (arrow) and not more posterior to this. (I–L) Pax2.1 expression (24 h). (I) In wild-type embryos, pax2.1 is expressed in the retina, midhindbrain border, otic vesicle, dorsal spinal cord, and pronephric mesoderm. (J) In ace mutant embryos, pax2.1 expression is absent from the midhindbrain border and is reduced in the retina and otic vesicle. (K) In Zoep mutant embryos with a strong phenotype, pax2.1 expression is absent from the retina, and is reduced in the otic vesicle. (L) Severely affected Zoep;ace mutant embryo; pax2.1-expressing cells are only detected in the anterior spinal cord (arrow). (M and N) Midsomitogenesis live embryos, anterior to left. Note the large numbers of opaque dead or dying cells in posterior tissues of the Zoep;ace embryo (N), which are not apparent in the wild-type embryos (M). |
Reprinted from Developmental Biology, 264(2), Griffin, K.J. and Kimelman, D., Interplay between FGF, one-eyed pinhead, and T-box transcription factors during zebrafish posterior development, 456-466, Copyright (2003) with permission from Elsevier. Full text @ Dev. Biol.